Abstract
Diabetic foot ulcers often become infected, leading to treatment complications and increased risk of loss of limb. Therapeutics to manage infection and simultaneously promote healing are needed. Here we report on the development of a Janus liposozyme that treats infections and promotes wound closure and re-epithelialization. The Janus liposozyme consists of liposome-like selenoenzymes for reactive oxygen species (ROS) scavenging to restore tissue redox and immune homeostasis. The liposozymes are used to encapsulate photosensitizers for photodynamic therapy of infections. We demonstrate application in methicillin-resistant Staphylococcus aureus-infected diabetic wounds showing high ROS levels for antibacterial function from the photosensitizer and nanozyme ROS scavenging from the liposozyme to restore redox and immune homeostasis. We demonstrate that the liposozyme can directly regulate macrophage polarization and induce a pro-regenerative response. By employing single-cell RNA sequencing, T cell-deficient Rag1−/− mice and skin-infiltrated immune cell analysis, we further reveal that IL-17-producing γδ T cells are critical for mediating M1/M2 macrophage transition. Manipulating the local immune homeostasis using the liposozyme is shown to be effective for skin wound repair and tissue regeneration in mice and mini pigs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All generated or analysed data supporting the findings of this study are available within the paper and its Supplementary Information. The RNA-seq data are available from the Gene Expression Omnibus (GEO) database, with accession number GSE238152. The single-cell RNA-seq data are available from the GEO database, with accession number GSE253098. All raw data from this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Rice, J. B. et al. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 37, 651–658 (2014).
Theocharidis, G. et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat. Commun. 13, 181 (2022).
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E. & Hicks, C. W. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221 (2023).
Zhang, P. et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann. Med. 49, 106–116 (2017).
Falanga, V. et al. Chronic wounds. Nat. Rev. Dis. Prim. 8, 50 (2022).
Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 366, 1736–1743 (2005).
Naghibi, M. et al. The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes. Diabetes Res. Clin. Pract. 4, 27–35 (1987).
Zykova, S. N. et al. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49, 1451–1458 (2000).
Thurlow, L. R., Stephens, A. C., Hurley, K. E. & Richardson, A. R. Lack of nutritional immunity in diabetic skin infections promotes Staphylococcus aureus virulence. Sci. Adv. 6, eabc5569 (2020).
Lavery, L. A. et al. Risk factors for foot infections in individuals with diabetes. Diabetes Care 29, 1288–1293 (2006).
Armstrong, D. G. et al. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 13, 16 (2020).
Geiss, L. S. et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 42, 50–54 (2019).
Boulton, A. J., Vileikyte, L., Ragnarson-Tennvall, G. & Apelqvist, J. The global burden of diabetic foot disease. Lancet 366, 1719–1724 (2005).
Jeffcoate, W. J., Vileikyte, L., Boyko, E. J., Armstrong, D. G. & Boulton, A. J. M. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 41, 645–652 (2018).
Bowling, F. L., Rashid, S. T. & Boulton, A. J. Preventing and treating foot complications associated with diabetes mellitus. Nat. Rev. Endocrinol. 11, 606–616 (2015).
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F. & Nogueira-Machado, J. A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell. Death. Dis. 9, 119 (2018).
Eming, S. A., Martin, P. & Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr266 (2014).
Zhang, Y. et al. Scarless wound healing programmed by core–shell microneedles. Nat. Commun. 14, 3431 (2023).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
Willenborg, S. et al. Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell. Metab. 33, 2398–2414 (2021).
Veves, A., Falanga, V., Armstrong, D. G., Sabolinski, M. L. & Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 24, 290–295 (2001).
Marston, W. A., Hanft, J., Norwood, P., Pollak, R. & Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701–1705 (2003).
Theocharidis, G. et al. A strain-programmed patch for the healing of diabetic wounds. Nat. Biomed. Eng. 6, 1118–1133 (2022).
Cruciani, M., Lipsky, B. A., Mengoli, C. & de Lalla, F. Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections?: A meta-analysis. Diabetes Care 28, 454–460 (2005).
Ziyadeh, N., Fife, D., Walker, A. M., Wilkinson, G. S. & Seeger, J. D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Skin. Wound Care. 24, 31–39 (2011).
Zhu, Y. et al. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl Acad. Sci. USA 115, 6816–6821 (2018).
Ren, J., Yang, M., Xu, F., Chen, J. & Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 233, 116728 (2019).
Chen, H. et al. Symbiotic algae–bacteria dressing for producing hydrogen to accelerate diabetic wound healing. Nano Lett. 22, 229–237 (2022).
Zhao, X. D. et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv. Funct. Mater. 31, 2009442 (2021).
Lipsky, B. A. et al. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 39, 885–910 (2004).
Kalelkar, P. P., Riddick, M. & Garcia, A. J. Biomaterial-based delivery of antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 7, 39–54 (2022).
Game, F. Management of osteomyelitis of the foot in diabetes mellitus. Nat. Rev. Endocrinol. 6, 43–47 (2010).
Xiu, W. et al. Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections. Nat. Commun. 13, 3875 (2022).
Yang, X. et al. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11, 5737–5745 (2017).
Gao, S. et al. Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated virus coat protein. Proc. Natl Acad. Sci. USA 116, 23437–23443 (2019).
Rotruck, J. T. et al. Selenium: biochemical role as a component of glutathione peroxidase. Science 179, 588–590 (1973).
Li, P. et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 22, 1107–1117 (2021).
Makabenta, J. M. V. et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36 (2021).
Garcia Soriano, F. et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat. Med. 7, 108–113 (2001).
Xu, H. et al. Notch–RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 13, 642–650 (2012).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Hu, W. et al. Skin γδ T cells and their function in wound healing. Front. Immunol. 13, 875076 (2022).
Heath, W. R. & Carbone, F. R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 14, 978–985 (2013).
Seraphim, P. M. et al. Lack of lymphocytes impairs macrophage polarization and angiogenesis in diabetic wound healing. Life Sci. 254, 117813 (2020).
Kleinert, M. et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162 (2018).
Maschalidi, S. et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes. Nature 606, 776–784 (2022).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Zhang, S. et al. Reversing SKI–SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature 551, 105–109 (2017).
Ye, Z. et al. Characterization of TGF-beta signaling in a human organotypic skin model reveals that loss of TGF-betaRII induces invasive tissue growth. Sci. Signal. 15, eabo2206 (2022).
Buechler, M. B., Fu, W. & Turley, S. J. Fibroblast–macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 54, 903–915 (2021).
Schatteman, G. C., Hanlon, H. D., Jiao, C., Dodds, S. G. & Christy, B. A. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J. Clin. Invest. 106, 571–578 (2000).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 5.47.1–5.47.20 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China project (81921004, D.K.; 82241218 and 31972896, S.Z.; 82372140, C.Z.), National Key Research and Development Program of China (2021YFA1201103, S.Z.) and Tianjin Natural Science Foundation (22JCZDJC00180, C.Z.; 22JCYBJC00460, D.L.) and we acknowledge the financial support from the Fundamental Research Funds for the Central Universities (63231049, D.L.; 63213080, C.Z.) and Fundamental Research Funds for Institute of Transplantation Medicine of Nankai University (NKTM2023003, S.Z.; NKTM2023004, C.Z.). We thank PETCC, the Global Pets’ Cell Resource Center, for kindly providing us with cell lines and medium for testing. We also thank the Flow Cytometry Core Facility, Microscopy Platform, Bioinformatics Platform and Mass Spectrometry Platform at the College of Life Sciences, Nankai University, for supporting our work.
Author information
Authors and Affiliations
Contributions
C.Z. and S.Z. designed the study. C.Z. conceived the idea and developed the materials and method for the Janus liposozyme. T.P. and X.M. synthesized seleno-phospholipid (Se-DOPE). Y.Z. and J.Q. designed and synthesized TDTM. T.W. and T.P. designed the in vitro and in vivo experiment. T.W., T.P., Y.G., X.M., J.Q. and Y.Z. characterized the Janus liposozyme in vitro. T.W., Y.G. and W.W. evaluated the antibacterial effect. T.W., X.M. and Y.G. designed and conducted the in vivo diabetic wound healing experiment and analysis in WT mice and Bama mini pigs. T.W., X.P., R.G., M.Z. and D.L. designed and conducted the in vivo diabetic wound healing experiment and analysis in genetic ablation of T cells in mice. X.P., R.G., F.K. and M.H. completed the flow cytometry experiment and analysis. T.W. and D.Z. performed histology assessment, immunofluorescence and RNA-seq analysis. X.P. and M.Z. conducted single-cell RNA-seq experiments. X.P., F.D., M.Z. and S.Z. contributed to single-cell RNA-seq analysis. T.W., X.P., M.Z., L.Z. and C.Z. prepared the figures. T.W., X.P., M.Z., D.K., S.Z. and C.Z. wrote the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
C.Z., T.P., X.M., T.W. and S.Z. are the inventors of a patent application (application no. 2024102459627) that covers the synthesis and antioxidative function of seleno-phospholipids. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Mingzhen Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
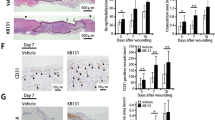
Extended Data Fig. 1 In vivo wound healing efficacy on db/db mice infected by MRSA.
a, Representative images of infected diabetic wounds of db/db mice in different treatment groups. Inside the green dashed line is the initial wound area. Scale bars, 2 mm. b, In vivo wound closure rates of db/db mice in different treatment groups. n = 5 biologically independent samples. c, Representative H&E staining images and quantitative analysis of length of regenerated epidermis in wounds of db/db mice in different treatment groups on day 15. Scale bars, 200 µm. n = 5 biologically independent samples. d, Representative Masson staining images in wounds of db/db mice in different treatment groups on day 15. Scale bars, 200 µm. e, Representative DHE staining images and quantitative analysis of DHE intensity in wounds of db/db mice in different treatment groups on day 15. Scale bars, 50 µm. n = 5 biologically independent samples. f, Representative α-SMA staining images and quantitative analysis of α-SMA+ vessel area in wounds of db/db mice in different treatment groups on day 15. Scale bars, 100 µm. n = 5 biologically independent samples. g, Representative CD31 staining images and quantitative analysis of capillary density in wounds of db/db mice in different treatment groups on day 15. Scale bars, 100 µm. n = 5 biologically independent samples. Control represents PBS buffer-treated group. All values are expressed as mean ± s. d. Statistical significance was determined using two-way ANOVA with Tukey’s multiple comparisons in b and two-tailed unpaired t test in c, e, f, g. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 2 In vivo wound healing efficacy on severe diabetic mice infected by MRSA.
a, Representative images of infected diabetic wounds of severe diabetic mice in different treatment groups. Inside the green dashed line is the initial wound area. Scale bars, 2 mm. b, In vivo wound closure rates of severe diabetic mice in different treatment groups. n = 5 biologically independent samples. c, Representative DHE staining images and quantitative analysis of DHE intensity in wounds of severe diabetic mice in different treatment groups on day 15. Scale bars, 50 µm. n = 5 biologically independent samples. d, Representative H&E staining images and quantitative analysis of length of regenerated epidermis in wounds of severe diabetic mice in different treatment groups on day 15. Scale bars, 200 µm. n = 5 biologically independent samples. e, Representative α-SMA staining images and quantitative analysis of α-SMA+ vessel area in wounds of severe diabetic mice in different treatment groups. Scale bars, 100 µm. n = 5 biologically independent samples. f, Representative CD68 and CD206 staining images and quantitative analysis of the ratio of CD206+/CD68+ cells in wounds of severe diabetic mice in different treatment groups. Scale bars, 50 µm. n = 5 biologically independent samples. Control represents PBS buffer-treated group. All values are expressed as mean ± s. d. Statistical significance was determined using two-way ANOVA with Tukey’s multiple comparisons in b and two-tailed unpaired t test in c-f. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21 and Tables 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, T., Pan, T., Peng, X. et al. Janus liposozyme for the modulation of redox and immune homeostasis in infected diabetic wounds. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01660-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01660-y
This article is cited by
-
Liposozyme for wound healing and inflammation resolution
Nature Nanotechnology (2024)