Abstract
With antibiotics resistance developing rapidly, new antibacterial agents are needed to be discovered. We readily synthesized 11 indolin-2-one compounds and found a hybrid of indolin-2-one and nitroimidazole 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one to be effective on Staphylococcus aureus strains. Six derivatives of this compound were further designed and synthesized in order to enhance its efficacy. After a second turn of structural refinement, a novel hybrid of indolin-2-one and nitroimidazole 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-5-nitroindolin-2-one with a nitro group on C-5 position of indolin-2-one was shown to exhibit remarkable antibacterial activities with a low MIC value against MRSA ATCC 33591. Besides, this molecule demonstrated its potency on Gram-negative bacteria and VRE strain. The time-killing curve experiment showed its good bactericidal activity. Low hemolytic rate suggested its promising safety profile.
Similar content being viewed by others
Introduction
The growing crisis of antibiotics has placed enormous threat upon mankind. Such crisis stems mainly from the resistance of pathogenic bacteria toward antibiotics. Among all those drug-resistant microbes, the notorious “ESKAPE pathogens,” a group of bacteria comprising Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, was believed to cause various hospital infections, and to be able to effectively “escape” the effects of multiple antibacterial drugs [1]. Furthermore, A. baumannii, P. aeruginosa, and Enterobacter species occupy the top three positions of WHO’s list of “the drug-resistant bacteria that pose the greatest threats to human health” in 2017 for their ability to resist the “last resort” antibiotics carbapenem [2]. Since the therapeutic options for these pathogens are increasingly limited due to multidrug resistance development, novel antibacterial agents with new antimicrobial profiles are desperately needed to be discovered.
Indolin-2-one is a chemical scaffold ubiquitous in nature, ranging from tissues and fluids of mammals to products produced by plants, bacteria, and invertebrates [3]. The indolin-2-one derivatives have been known to possess a broad spectrum of biological and pharmacological activities, including kinase inhibitory activity [4, 5], anticancer [6, 7], anti-Alzheimer’s [8, 9], antinociceptive, anti-inflammatory [10, 11], antioxidant [12, 13], neuroprotective [14, 15], and antibacterial activity [16, 17]. Derivatives with 3-(substitutedmethylene)indolin-2-one scaffold have been reported to exhibit inhibitory activities against various bacteria. Compound A (violacein, Fig. 1) was implicated to interfere S. aureus planktonic cells and biofilms [18]. Compound B (Fig. 1) has effect both on two Gram-negative bacteria, Escherichia coli, P. aeruginosa, and on two Gram-positive bacteria, Streptococcus pyogenes and S. aureus [19]. Compound C [20], compound D [21], and compound E [22] are able to inhibit growth of Agrobacterium tumefaciens, Erythrobacter litoralis, and Candida albicans, respectively (Fig. 1). These instances imply 3-(substitutedmethylene)indolin-2-one as a promising antibacterial scaffold.
Although a large number of molecules with 3-(substitutedmethylen)indolin-2-one skeleton has been depicted to exhibit antibacterial activities, but to the best of our knowledge these reported compounds exhibit minimum inhibitory concentration (MIC) values mainly ranging from 10 to 64 μg/mL against reported bacteria, none of these molecules are able to enter the drug development pipeline. Herein, with the intention of discovering new indolin-2-one antibiotics and disclosing the antibacterial structure-activity relationship (SAR) of this scaffold, we investigated the antibacterial properties of a class of 3-(substitutedmethylene)indolin-2-one compounds, and presented one indolin-2-one compound possessing a nitroimidazole moiety with a remarkable MIC of 0.0625–0.125 μg/mL against methicillin-resistant S. aureus (MRSA) strain ATCC 33591.
Results and discussion
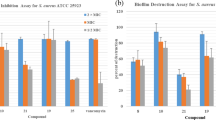
Aiming at seeking effective antibacterial indolin-2-one agents, we firstly synthesized 11 different 3-(substitutedmethylene)indolin-2-ones, and screened their antibacterial activities on S. aureus strains. A hybrid of indolin-2-one and nitroimidazole 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one (3g) was found to exhibit a MIC of 2 μg/mL and 1 μg/mL on methicillin-sensitive S. aureus (MSSA) ATCC 25923 and MRSA ATCC 33591, respectively. Then we investigated the initial SAR of this chemical class, and found one compound, 3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-5-nitroindolin-2-one (5c), to possess a high potency of antibacterial activity with a MIC of 0.0625–0.125 μg/mL against MRSA strain ATCC 33591; we also found this molecule to be effective on two Gram-negative bacteria strains E. coli ATCC 25922, P. aeruginosa ATCC 27853 and one Gram-positive bacteria strain vancomycin-resistant Enterococcus (VRE) strain B148, respectively. The time-kill curve experiment was performed to test the bactericidal activity of compound 5c on MSSA ATCC 6538, MRSA ATCC 33591, and E. coli ATCC 25922; a total eradication of bacteria cells at a concentration of 16* MIC of 5c was observed among all three strains. Next, the hemolytic rates of 5c and 3g were determined and low hemolytic rates were observed, suggesting a promising safety profile of this chemical class.
Synthesis and biological evaluation
At the beginning of the study, a one-step simple synthetic strategy (Scheme 1) was used to quickly obtain several 3-(arylmethylene)indolin-2-ones. The synthesis was carried out by aldol condensation using 1,3-dihydro-2H-indole-2-one with 11 aromatic aldehydes in the presence of piperidine in methyl alcohol to afford 11 corresponding 3-substituted indolin-2-ones 3a–3k (Table 1). Various aldehydes with different aromatic rings were chosen for SAR investigation, including benzpyrole, anthracene, phenanthrene, quinoline, pyridine, pyrrole, furan, nitrobenzene, nitroimidazole, and nitrothiophene. The Z/E configuration of each compound was determined by their 2D ROESY NMR spectrum (Supporting Information), investigating whether the olefinic hydrogen has a co-signal with hydrogen at C-4 position of indolin-2-one, or the C-4 position hydrogen has a co-signal with hydrogens on aromatic ring of aldehyde moiety (Fig. 2). The reported compound 3g, of which the configuration was not disclosed in the literature [23], along with the compounds 3a–3b, 3d–3f, 3j, and5a–5f adopted an E configuration, compounds 3h, 3i, and 3k a Z one, while 3c was a Z/E mixture. With eleven 3-(argiomethylene)indolin-2-ones in hand, we tested their antibacterial activities against both MRSA and MSSA strains. The MICs of these compounds (Table 1) are determined by broth microdilution method (see Experimental Procedures for detail). Compound 3g, a hybrid of indolin-2-one and nitroimidazole, exhibited a low MIC of 2 μg/mL and 0.5–1 μg/mL against MSSA ATCC 25923 and MRSA ATCC 33591, respectively. Compound 3d showed a MIC of 16 μg/mL on MSSA ATCC 25923, while its MIC on MRSA ATCC 33591 was higher than 64 μg/mL. Other compounds exerted MIC values higher than 64 μg/mL on both stains.
The SAR study above indicated that nitroimidazole moiety plays an essential role in enhancing the antibacterial activities of indolin-2-ones. This pharmacophore is widely known for its ability to combat a broad spectrum of Gram-positive and Gram-negative bacteria, such as Clostridium difficile and Bacteroides fragilis [24]. We consequently wondered whether compound 3g has an effect on Gram-negative bacteria and other drug-resistant bacteria or not. Two clinical related Gram-negative strains, E. coli ATCC 25922 and P. aeruginosa ATCC 27853, and one Gram-positive drug-resistant bacteria strain, VRE B148, were chosen and treated with 3g, but no MICs below 64 μg/mL were observed (Table 1).
To disclose the SAR of 3g as well as to discover more effective antibacterial agents, six hybrids (5a–5f) of indolin-2-one and nitroimidazole with various substituents on the benzene ring of indolin-2-one were prepared. Compounds 5a–5f were obtained from aldol condensation of compounds 4a–4f with 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) (Scheme 2). Compounds 4a–4c and 4e were commercially available, while compounds 4d and 4f were synthetic intermediates. Compound 4d was obtained by condensing 3-(piperidin-1-yl)propanoic acid with intermediate 6, which was obtained by reduction of compound 4c (Scheme 2). Compound 4f was synthesized starting from compound 1, after substitution with chlorosulfonic acid at C-5 position to afford intermediate 7 and condensing with morpholine; the intermediate 4f thus formed was finally reacted with 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to give compound 5f (Scheme 2). All of these six hybrids of indolin-2-one and nitroimidazole adopted an E configuration (Supporting Information). The MIC values against ATCC 33591 and ATCC 25923 of these six molecules were screened (Table 1).
Introduction of a nitro group at either C-5 position (5c) or C-6 position (5e) of the phenyl ring of indolin-2-one significantly increased the antibacterial activities of this class; a 16-fold and 8-fold increase in efficacy of 5c (compared with 3g) against ATCC 25923 and ATCC 33591 were found, while 5e exhibited a fourfold and twofold increased efficacy (compared with 3g) against ATCC 25923 and ATCC 33591, respectively. A derivative with a 3-(piperidin-1-yl)propanamide substituent (5d) at C-5 position resulted in a fourfold drop in potency on both stains. However, introduction of a trifluoromethyl moiety, a bromine atom, and a morpholinosulfonyl moiety almost completely abolished inhibitory activities. Interestingly, compound 5c also showed potential activity against two Gram-negative bacteria strains and VRE strain mentioned above (Table 1). These initial SAR studies showed great antibacterial potential of hybrids of indolin-2-one and nitroimidazole; especially, introduction of a nitro group on indolin-2-one remarkably enhanced the antimicrobial activities as well as broadened the antibacterial spectrum.
we also found this molecule to be effective on two Gram-negative bacteria strains E. coli ATCC 25922, P. aeruginosa ATCC 27853 and one Gram-positive bacteria strain vancomycin-resistant Enterococcus (VRE) strain B148, respectivelyThe most effective compound 5c was experimented for its time-kill ability. The time-kill curve experiments (see Experimental Procedures for detail) were performed at concentrations of 1/4* MIC, 1* MIC, 4* MIC, and 16* MIC of compound 5c against two S. aureus strains, MSSA ATCC 6538, MRSA ATCC 33591, and one E. coli strain, ATCC 25922. When treated with 16* MIC compound concentration of 5c, bacterial growth was absent among all three strains, while 1/4* MIC of 5c almost did not kill any microbes. A slight bacterial regrowth phenomenon was observed after 4–6 h of incubation with bacteria with 1* MIC or 4* MIC of 5c within three strains, especially in E. coli (Fig. 3). Furthermore, compound 3g and 5c were tested for their hemolytic activities (see Experimental Procedures for detail), both 3g and 5c showed significant low hemolytic rates even at 500 μM (Fig. 4), indicating a promising safety profile.
Conclusion
In conclusion, we have discovered a chemical class of hybrids of indolin-2-one and nitroimidazole that possessed a high potency of killing several bacteria strains, including drug-resistant bacteria. The initial SAR study showed great potential in development of this class, and the low hemolytic rates suggested a promising safety profile for this scaffold. The introduction of the nitro group resulted in significant enhancement of potency and broad antibacterial spectrum of this class. This result suggested the presumption that compound 5c may have an effect on the nitroreductase of bacteria, and our further work should concentrate on proof of this hypothesis. This study presented a preliminary insight into this chemical structure; we consider that our further research on this class of compounds should consist in exploration of the mechanism of action and investigation of the pharmacodynamics and pharmacokinetics of the hit compound.
Experimental procedures
Materials, bacteria strains, and general instruments
All reagents were directly used as purchased without further purification. All solvents were dried according to standard methods before use, solvents used in optical spectroscopic studies were HPLC grade. 1H NMR, 13C NMR and 2D ROSEY NMR spectrum were recorded on a Bruker Avance (Varian Unity Inova) 500 MHz spectrometer in DMSO-d6 with tetramethylsilane (TMS) as internal standard. 1H NMR and 13C NMR were analyzed by MestReNova Software, while 2D ROSEY was analyzed by Topspin Software. High resolution mass spectrum (HRMS) was performed on an Agilent LC/MSD TOF system G3250AA. Analytical thin layer chromatography (TLC) was performed on silica gel 60 F254 precoated plates (0.25 mm) from Qingdao Haiyang Inc., and components were visualized by ultraviolet light (254 nm). Silicycle silica gel 300−400 (particle size 40−63 μm) mesh was used for all flash column chromatography experiments. Bacteria strains MRSA ATCC 33591, MSSA ATCC 25923, MSSA ATCC 6538, E. coli ATCC 25922, P. aeruginosa ATCC 27853 were purchased from the American Type Culture Collection, bacteria strain VRE B148 was isolated from clinical patients. The sheep erythrocytes were purchased from Hopebio, China.
Measuring of MICs
The MICs were determined in triplicate for each compound by broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The organisms were grown in Ca2+-supplemented and Mg2+-supplemented Mueller-Hinton broth (MHB) at a final inoculum of 5 × 105 CFU/mL. Compounds were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) to a concentration of 12.8 mg/mL. MICs were determined after 20 h of incubation at 35 °C in ambient air.
Time-kill curve experiments
All time-kill methods, at an inoculum of approximately 5 × 105 CFU/mL, were performed with 1/4* MIC to 16* MIC of the respective compound for 24 h. Samples (100 μL) were taken at 0, 2, 4, 6, 8, and 24 h, consequently diluted with 0.9% sodium chloride solution, and plated on Luria-Bertani (LB) agar. All plates were incubated at 37 °C for 24 h, and determinations of the numbers of colony-forming units (CFU) were based on plates with 30–300 colonies per plate. All experiments were performed in triplicate. Absence of growth in 2/3 experiments was regarded as complete kill.
Measuring of hemolytic rates
Briefly, sheep erythrocytes were washed three times with 10 mM Tris-HCl, 0.9% NaCl pH 7.4 and re-suspended to a final concentration of 2%. A total of 190 μL of erythrocytes was mixed with 10 μL of the serially twofold diluted testing compounds and incubated at 37 °C for 1 h. Furthermore, 10 mM Tris-HCl, 0.9% NaCl (pH 7.4), and Milli-Q water were used as negative and positive controls (the hemolytic rate of Milli-Q water was set as 100%), respectively. After incubation, the erythrocytes solution was centrifuged for 450 g, 10 mins, and absorbance of the supernatant was measured at 545 nm.
General procedure α for the synthesis of compounds 3a–3k
To a solution of indolin-2-one (compound 1) (200 mg, 1 eq.) in MeOH (5 mL), piperidine (1.5 eq.), and corresponding aldehydes (2a–2k) (1.2 eq.) were added. The reaction mixture was heated to reflux and stirred for 1–4 h, then cooled to room temperature. The mixture was filtered, and the filter cake was washed with MeOH three times, then the filter cake was collected and dried under vacuum to remove MeOH to give the desired compounds 3a–3k.
Synthesis of intermediate 4d
To a solution of 3-(piperidin-1-yl)propanoic acid (600 mg, 1 eq.) in dichloromethane (DCM) (7 mL), triethylamine (1.4 eq.), and 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (1.3 eq.) were added. The reaction mixture was stirred at room temperature for 20–30 min, then 5-aminoindolin-2-one (1.1 eq.) was added into the reaction mixture. The resulting mixture was stirred at room temperature for 6 h. After the reaction was finished, the solvent was evaporated under vacuum, then purified by column chromatography to give intermediate 4d as a colorless oil (yield 33.4%).
Synthesis of intermediate 4f
To a suspension of compound 7 (250 mg, 1 eq.) in DCM (7 mL) was added morpholine (1.5 eq.). The reaction mixture was stirred for 2 h at room temperature. After the reaction finished, the mixture was washed with brine for three times, and the organic layer was concentrated under vacuum to afford crude product, which was purified by column chromatography to give intermediate 4f as a white solid (yield 75.8%).
General procedure β for the synthesis of compounds 5a–5f
To a solution of the corresponding compounds 4a–4f (200 mg, 1 eq.) in MeOH (5 mL), piperidine (1.5 eq.) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) (1.2 eq.) were added. The reaction mixture was heated to reflux and stirred for 1–4 h, then cooled to room temperature. The mixture was filtered and the filter cake was washed with MeOH three times, then the filter cake was collected and dried under vacuum to remove MeOH to give the desired compounds 5a–5f.
Synthesis of intermediate 6
To a suspension of 5-nitroindolin-2-one (5 g, 1 eq.) in EtOH (50 mL), activated carbon (1 g) and FeCl3 (1 g) were added. The mixture was heated to 78 °C, and stirred for 10 min. Then 80% aqueous solution of hydrazine hydrate (8 eq.) was added dropwise into the reaction mixture in 5 min, the resulting mixture was stirred at 78 °C for 8–10 h, then cooled to room temperature. The mixture was filtered to remove residue of activated carbon; the filtrate was concentrated under vacuum to afford crude product, which was purified by recrystallization from EtOH (about 15 mL) to give 5-aminoindolin-2-one as a pale yellow solid (yield 91.9%).
Synthesis of intermediate 7
In a round bottle vessel was added chlorosulfonic acid (3 mL) dropwise to compound 1 (500 mg) at 0 °C. The mixture was stirred for 30 min then was heated to 70 °C and reacted for 1 h and cooled to room temperature. The resulting mixture was added dropwise into ice water and then filtered, the filter cake was washed with water for three times and dried to give intermediate 7 as a pink solid (yield 98.3%)
(E)-3-(anthracen-9-ylmethylene)indolin-2-one (3a)
Following general procedure α, starting from indolin-2-one (1) and anthracene-9-carbaldehyde (2a) to afford compound 3a as an E isomer, yellow solid (250 mg, yield 51.7%). 1H NMR (400 MHz, DMSO-d6) δ 10.75 (s, 1H), 8.78 (s, 1H), 8.35 (s, 1H), 8.21 (d, J = 8.0 Hz, 2H), 7.99 (d, J = 8.4 Hz, 2H), 7.56 (m, 4H), 7.07 (t, J = 7.6 Hz, 1H), 6.85 (d, J = 8.0 Hz, 1H), 6.38 (t, J = 7.6 Hz, 1H), 5.66 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 168.24, 143.57, 132.80, 131.85, 131.27 (2C), 130.57, 129.51 (2C), 128.87, 128.68 (2C), 128.62, 127.29 (2C), 126.26 (2C), 125.62 (2C), 123.66, 121.51, 121.48, 110.39.
(E)-3-(phenanthren-9-ylmethylene)indolin-2-one (3b)
Following general procedure α, starting from indolin-2-one (1) and phenanthrene-9-carbaldehyde (2b) to afford compound 3b as an E isomer, yellow solid (445 mg, yield 92.2%). 1H NMR (400 MHz, DMSO-d6) δ 10.70 (s, 1H), 8.97 (d, J = 8.4 Hz, 1H), 8.92 (d, J = 8.4 Hz, 1H), 8.18 (s, 1H), 8.11 (s, 1H), 8.06 (d, J = 7.6 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.79 (m, 2H), 7.71 (m, 2H), 7.17 (t, J = 7.6 Hz, 1H), 6.97 (d, J = 7.6 Hz, 1H), 6.89 (d, J = 8.0 Hz, 1H), 6.67 (t, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 168.71, 143.59, 133.91, 131.04, 130.95, 130.71, 130.63, 130.52, 130.45, 129.85, 129.53, 128.42, 128.03, 127.96, 127.94, 127.87, 125.65, 124.11, 123.50, 123.23, 121.64, 121.57, 110.62. HRMS (Q-TOF): calculated for C23H15NO [M]: 321.1154. Found [M + H]+: 322.1250.
(Z/E)-3-(4-nitrobenzylidene)indolin-2-one (3c)
Following general procedure α, starting from indolin-2-one (1) and 4-nitrobenzaldehyde (2c) to afford compound 3c as a Z/E mixture (Z/E = 1/2), yellow solid (246 mg, yield 61.5%). 1H NMR (400 MHz, DMSO-d6) δ 10.71 (m, 1/3HZ + 2/3HE), 8.50 (m, 2/3HZ), 8.35 (m, 4/3HE), 8.28 (m, 2/3HZ), 7.95 (m, 4/3HE), 7.91 (s, 1/3HZ), 7.75 (d, J = 7.6 Hz, 1/3HZ), 7.67 (s, 2/3HE), 7.41 (d, J = 8.0 Hz, 2/3HE), 7.27 (m, 1/3HZ + 2/3HE), 7.02 (t, J = 7.6 Hz, 1/3HZ), 6.87 (m, 2/3HZ + 1HE). 13C NMR (100 MHz, DMSO-d6) δ 168.62, 167.24, 147.92, 147.86, 143.98, 142.03, 141.93, 140.72, 133.90, 133.35, 132.93, 131.48, 130.92, 130.62, 130.56, 124.69, 124.37, 123.59, 123.35, 121.84, 121.14, 120.75, 110.85, 110.14.
(E)-3-(pyridin-2-ylmethylene)indolin-2-one (3d)
Following general procedure α, starting from indolin-2-one (1) and picolinaldehyde (2d) to afford compound 3d as an E isomer, yellow solid (215 mg, yield 64.4%). 1H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 9.00 (d, J = 7.6 Hz, 1H), 8.89 (m, 1H), 7.96 (m, 1H), 7.87 (m, 1H), 7.57 (s, 1H), 7.47 (m, 1H), 7.29 (m, 1H), 6.99 (m, 1H), 6.87 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.76, 153.72, 150.14, 144.09, 137.72, 134.13, 131.30, 129.77, 128.88, 128.41, 124.60, 122.00, 121.70, 110.08.
(E)-3-((5-iodofuran-2-yl)methylene)indolin-2-one (3e)
Following general procedure α, starting from indolin-2-one (1) and 5-iodofuran-2-carbaldehyde (2e) to afford compound 3e as an E isomer, yellow solid (470 mg, yield 92.8%). 1H NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H), 8.27 (d, J = 8.0 Hz, 1H), 7.28 (m, 2H), 7.18 (d, J = 3.6 Hz, 1H), 7.05 (m, 2H), 6.90 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.58, 155.98, 143.25, 130.42, 124.73, 124.19, 123.13, 123.02, 121.77, 121.65, 118.48, 110.34, 99.22.
(E)-3-((1H-indol-7-yl)methylene)indolin-2-one (3f)
Following general procedure α, starting from indolin-2-one (1) and 1H-indole-7-carbaldehyde (2f) to afford compound 3f as an E isomer, yellow solid (172 mg, yield 44.0%). 1H NMR (400 MHz, DMSO-d6) δ 11.43 (s, 1H), 10.60 (s, 1H), 7.98 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.53 (d, J = 7.2 Hz, 1H), 7.39 (t, J = 2.8 Hz, 1H), 7.31 (d, J = 7.6 Hz, 1H), 7.20 (td, J = 7.6, 0.8 Hz, 1H), 7.15 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.78 (td, J = 7.6, 0.8 Hz, 1H), 6.56 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.27, 143.26, 134.63, 132.45, 130.27, 128.90, 128.13, 126.68, 122.89, 122.84, 122.08, 121.90, 121.44, 119.19, 118.77, 110.38, 102.33.
(E)-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one (3g)
Following general procedure α, starting from indolin-2-one (1) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 3g as an E isomer, orange solid (280 mg, yield 69.0%). 1H NMR (400 MHz, DMSO-d6) δ 10.73 (s, 1H), 9.01 (d, J = 7.6 Hz, 1H), 8.42 (s, 1H), 7.33 (m, 2H), 7.00 (td, J = 7.6, 1.2 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 4.08 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.99, 146.89, 144.53, 140.13, 134.03, 132.67, 132.40, 128.41, 121.93, 121.03, 116.62, 110.37, 34.04.
(Z)-3-((3,5-dimethyl-1H-pyrrol-2-yl)methylene)indolin-2-one (3h)
Following general procedure α, starting from indolin-2-one (1) and 3,5-dimethyl-1H-pyrrole-2-carbaldehyde (2h) to afford compound 3h as a Z isomer, yellow solid (308 mg, yield 86.0%). 1H NMR (400 MHz, DMSO-d6) δ 13.35 (s, 1H), 10.76 (s, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.55 (s, 1H), 7.09 (td, J = 7.6, 1.2 Hz, 1H), 6.97 (td, J = 7.6, 1.2 Hz, 1H), 6.87 (m, 1H), 6.00 (d, J = 2.4 Hz, 1H), 2.32 (s, 3H), 2.30 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 169.87, 138.59, 136.04, 132.01, 127.11, 126.35, 126.18, 123.81, 121.29, 118.52, 113.20, 112.96, 109.72, 13.98, 11.78.
(Z)-3-((5-nitrothiophen-2-yl)methylene)indolin-2-one (3i)
Following general procedure α, starting from indolin-2-one (1) and 5-nitrothiophene-2-carbaldehyde (2i) to afford compound 3i as a Z isomer, dark brown solid (213 mg, yield 52.1%). 1H NMR (400 MHz, DMSO-d6) δ 10.90 (s, 1H), 8.18 (s, 1H), 8.15 (d, J = 4.4 Hz, 1H), 7.74 (m, 6.0 Hz, 2H), 7.30 (t, J = 7.6 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.90 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 167.79, 153.69, 143.46, 142.10, 136.61, 130.94, 129.27, 127.61, 127.15, 123.78, 122.03, 121.17, 110.56.
(E)-3-(quinolin-4-ylmethylene)indolin-2-one (3j)
Following general procedure α, starting from indolin-2-one (1) and quinoline-4-carbaldehyde (2j) to afford compound 3j as an E isomer, pale yellow solid (344 mg, yield 84.1%). 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1 H), 9.03 (d, J = 4.4 Hz, 1H), 8.15 (m, 1H), 7.99 (s, 1H), 7.95 (m, 1H), 7.86 (m, 1H), 7.75 (d, J = 4.0 Hz, 1H), 7.65 (m, 1H), 7.20 (m, 1H), 6.88 (d, J = 8.0 Hz, 1H), 6.68 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.20, 150.79, 148.37, 143.88, 141.30, 132.16, 131.30, 130.59, 130.56, 130.26, 127.89, 125.59, 125.30, 123.53, 121.77, 120.96, 120.92, 110.76.
(Z)-3-((5-methoxy-1H-indol-3-yl)methylene)indolin-2-one (3k)
Following general procedure α, starting from indolin-2-one (1) and 5-methoxy-1H-indole-3-carbaldehyde (2k) to afford compound 3k as a Z isomer, red solid (404 mg, yield 92.6%). 1H NMR (400 MHz, DMSO-d6) δ 11.89 (s, 1H), 10.49 (s, 1H), 9.43 (d, J = 2.0 Hz, 1H), 8.16 (s, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.74 (d, J = 2.4 Hz, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.14 (td, J = 7.6, 0.8 Hz, 1H), 6.99 (td, J = 7.6, 0.8 Hz, 1H), 6.87 (m, 2H), 3.89 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.61, 155.51, 139.47, 134.47, 131.22, 129.56, 128.07, 127.01, 126.34, 120.82, 119.18, 118.82, 113.42, 112.85, 111.86, 109.37, 101.21, 56.09.
N-(indolin-2-one-5-yl)-3-(piperidin-1-yl)propanamide (4d)
Colorless oil, yield 33.4%. 1H NMR (400 MHz, DMSO-d6) δ 10.27 (s, 1H), 10.03 (s, 1H), 7.50 (d, J = 0.8 Hz, 1H), 7.31 (dd, J = 8.4, 1.6 Hz, 1H), 6.74 (d, J = 8.4 Hz, 1H), 3.45 (s, 2H), 2.77 (m, 2H), 2.56 (m, 6H), 1.56 (m, 4H), 1.43 (m, 2H).
5-(Morpholinosulfonyl)indolin-2-one (4f)
White solid, yield 75.8%. 1H NMR (400 MHz, DMSO-d6) δ 10.86 (s, 1H), 7.56 (m, 2H), 7.03 (d, J = 8.4 Hz, 1H), 3.62 (m, 6H), 2.83 (m, 4H).
(E)-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-6-(trifluoromethyl)indolin-2-one (5a)
Following general procedure β, starting from 6-(trifluoromethyl)indolin-2-one (4a) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5a as an E isomer, yellow solid (276 mg, yield 82.1%). 1H NMR (400 MHz, DMSO-d6) δ 11.00 (s, 1H), 9.19 (d, J = 8.0 Hz, 1H), 8.42 (s, 1H), 7.44 (s, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.07 (s, 1H), 4.10 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.65, 146.22, 144.82, 140.35, 133.98, 131.44, 130.84, 128.71, 124.58, 124.34, 119.61, 118.65, 106.38, 34.13. HRMS (Q-TOF): calculated for C14H9FN4O3 [M]: 338.0627. Found [M + H]+: 339.0965.
(E)-5-bromo-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one (5b)
Following general procedure β, starting from 5-bromoindolin-2-one (4b) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5b as an E isomer, yellow solid (253 mg, yield 76.8%). 1H NMR (400 MHz, DMSO-d6) δ 10.88 (s, 1H), 9.24 (d, J = 2.0 Hz, 1H), 8.49 (s, 1H), 7.51 (dd, J = 8.4, 2.0 Hz, 1H), 7.40 (s, 1H), 6.86 (d, J = 8.4 Hz, 1H), 4.10 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.59, 146.49, 143.48, 140.29, 134.59, 134.08, 131.33, 130.48, 122.99, 118.36, 113.65, 112.29, 34.15. HRMS (Q-TOF): calculated for C13H9BrN4O3 [M] :347.9858. Found [M + H]+: 348.9931.
(E)-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-5-nitroindolin-2-one (5c)
Following general procedure β, starting from 5-nitroindolin-2-one (4c) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5c as an E isomer, yellow solid (266 mg, yield 75.2%). 1H NMR (400 MHz, DMSO-d6) δ 11.37 (s, 1H), 9.93 (d, J = 2.0 Hz, 1H), 8.47 (s, 1H), 8.23 (dd, J = 8.8, 2.4 Hz, 1H), 7.44 (s, 1H), 7.03 (d, J = 8.8 Hz, 1H), 4.12 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 169.25, 149.89, 146.14, 142.49, 140.46, 134.00, 130.27, 128.41, 123.68, 121.20, 119.53, 110.46, 34.20. HRMS (Q-TOF): calculated for C13H9N5O5 [M]:315.0604. Found [M + H]+: 317.1272.
(E)-N-(3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)indolin-2-one-5-yl)-3-(piperidin-1-yl)propanamide (5d)
Following general procedure β, starting from N-(2-oxoindolin-5-yl)-3-(piperidin-1-yl)propanamide (4d) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5d as an E isomer, brown solid (209 mg, yield 70.7%). 1H NMR (400 MHz, DMSO-d6) δ 10.66 (s, 1H), 10.18 (s, 1H), 8.94 (d, J = 1.2 Hz, 1H), 8.26 (s, 1H), 7.69 (dd, J = 8.4, 2.0 Hz, 1H), 7.33 (s, 1H), 6.82 (d, J = 8.4 Hz, 1H), 4.08 (s, 3H), 2.64 (m, 2H), 2.47 (m, 6H), 1.54 (m, 4H), 1.42 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 170.30, 168.98, 146.84, 140.35, 140.13, 133.75, 133.58, 133.13, 124.27, 121.02, 120.33, 117.02, 110.12, 54.78, 54.00 (2C), 34.10, 34.00, 25.97 (2C), 24.36. HRMS (Q-TOF): calculated for C21H24N6O4 [M]: 424.1859. Found [M + H]+: 425.1935.
(E)-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-6-nitroindolin-2-one (5e)
Following general procedure β, starting from 6-nitroindolin-2-one (4e) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5e as an E isomer, yellow solid (290 mg, yield 82.0%). 1H NMR (400 MHz, DMSO-d6) δ 11.14 (s, 1H), 9.26 (d, J = 8.4 Hz, 1H), 8.44 (s, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.54 m, 2H), 4.13 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.63, 149.21, 145.99, 145.06, 140.54, 134.04, 130.16, 128.68, 126.93, 121.19, 117.24, 104.61, 34.24. HRMS (Q-TOF): calculated for C13H9N5O5 [M] :315.0604. Found [M + H]+: 317.1280.
( E )-3-((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)-5-(morpholinosulfonyl) indolin-2-one (5f)
Following general procedure β, starting from 5-(morpholinosulfonyl)indolin-2-one (4f) and 1-methyl-5-nitro-1H-imidazole-2-carbaldehyde (2g) to afford compound 5f as an E isomer, yellow solid (231 mg, yield 77.8%). 1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H), 9.55 (d, J = 2.0 Hz, 1H), 8.48 (s, 1H), 7.72 (dd, J = 8.4, 2.0 Hz, 1H), 7.49 (s, 1H), 7.11 (d, J = 8.4 Hz, 1H), 4.12 (s, 3H), 3.65 (m, 4H), 2.94 (m, 4H). 13C NMR (100 MHz, DMSO-d6) δ 169.06, 148.13, 146.39, 140.46, 134.02, 132.14, 130.92, 127.98, 127.67, 121.47, 119.22, 110.60, 65.89 (2C), 46.44 (2C), 34.20. HRMS (Q-TOF): calculated for C17H17N5O6S [M]: 419.0900. Found [M + H]+: 420.0972.
5-Aminoindolin-2-one (6)
Pale yellow solid, yield 91.9%. 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 6.49 (m, 2H), 6.38 (m, 1H), 4.62 (s, 2H).
5-Chlorosulfonyl-indolin-2-one (7)
Pink solid, yield 98.3%. 1H NMR (400 MHz, DMSO-d6) δ 10.43 (s, 1H), 7.44 (m, 2H), 6.73 (d, J = 7.6 Hz, 1H), 3.47 (s, 2H).
References
Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12.
Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15.
Kaur M, Singh M, Chadha N, Silakari O. Oxindole: a chemical prism carrying plethora of therapeutic benefits. Eur J Med Chem. 2016;123:858–94.
Rajesh Kumar M,Alagumuthu M,Violet Dhayabaran V, N-substituted hydroxynaphthalene imino-oxindole derivatives as new class of PI3-kinase inhibitor and breast cancer drug: molecular validation and structure-activity relationship studies. Chem Biol Drug Des. 2018;91:277–84.
Ho HK, et al. Benzylidene-indolinones are effective as multi-targeted kinase inhibitor therapeutics against hepatocellular carcinoma. Mol Oncol. 2014;8:1266–77.
Ji C, et al. Design, synthesis and biological evaluation of novel antitumor spirotetrahydrothiopyran–oxindole derivatives as potent p53-MDM2 inhibitors. Bioorg Med Chem. 2017;25:5268–77.
Romagnoli R, et al. Design, synthesis and biological evaluation of 3-substituted-2-oxindole hybrid derivatives as novel anticancer agents. Eur J Med Chem. 2017;134:258–70.
Ashraf Ali M, et al. AChE inhibitor: a regio- and stereo-selective 1,3-dipolar cycloaddition for the synthesis of novel substituted 5,6-dimethoxy spiro[5.3′]-oxindole-spiro-[6.3″]-2,3-dihydro-1H-inden-1″-one-7-(substituted aryl)-tetrahydro-1H-pyrrolo[1,2-c][1,3]thiazole. Bioorg Med Chem Lett. 2012;22:508–11.
Akrami H, et al. Indolinone-based acetylcholinesterase inhibitors: synthesis, biological activity and molecular modeling. Eur J Med Chem. 2014;84:375–81.
Sun Y, et al. One-step synthesis of chiral oxindole-type analogues with potent anti-inflammatory and analgesic activities. Sci Rep. 2015;5:13699.
Chen G, et al. Synthesis and biological evaluation of novel indole-2-one and 7-aza-2-oxindole derivatives as anti-inflammatory agents. Drug Des Devel Ther. 2014; 8: 1869–92.
MIDOH N, et al. Antioxidative activities of oxindole-3-acetic acid derivatives from supersweet corn powder. Biosci Biotechnol Biochem. 2010;74:1794–801.
Ahmad I, et al. Xanthine oxidase/tyrosinase inhibiting, antioxidant, and antifungal oxindole alkaloids from Isatis costata. Pharm Biol. 2010;48:716–21.
Furuta K, Mizuno Y, Maeda M, Koyama H, Hirata Y. Synthesis of 3-arylmethyl-2-oxindole derivatives and their effects on neuronal cell death. Chem Pharm Bull. 2017;65:1093–7.
Furuta K, et al. Synthesis of 3-[4-(dimethylamino)phenyl]alkyl-2-oxindole derivatives and their effects on neuronal cell death. Bioorg Med Chem Lett. 2017;27:4457–61.
Gholamzadeh P, Mohammadi Ziarani G, Badiei A, Abolhassani Soorki A, Lashgari N. Efficient green synthesis of isoindigo derivatives using sulfonic-acid-functionalized nanoporous silica (SBA-Pr-SO3H) catalyst and study of their antimicrobial properties. Res Chem Intermed. 2013;39:3925–36.
Hosseinzadeh N, et al. 5-Nitro-heteroarylidene analogs of 2-thiazolylimino-4-thiazolidinones as a novel series of antibacterial agents. Med Chem Res. 2013;22:2293–302.
Batista A, et al. Antimicrobial effects of violacein against planktonic cells and biofilms of Staphylococcus aureus. Molecules. 2017;22:1534.
Rindhe SS, Karale BK, Gupta RC, Rode MA. Synthesis, antimicrobial and antioxidant activity of some oxindoles. Indian J Pharm Sci. 2011;73:292–6.
Li M-C, et al. Four new minor brominated indole related alkaloids with antibacterial activities from Laurencia similis. Bioorg Med Chem Lett. 2016;26:3590–3.
Majik MS, Rodrigues C, Mascarenhas S, D’Souza L. Design and synthesis of marine natural product-based 1H-indole-2,3-dione scaffold as a new antifouling/antibacterial agent against fouling bacteria. Bioorg Chem. 2014;54:89–95.
Acharya AP, et al. Green method for synthesis of 3-[2-(substituted-phenyl)-2-oxo ethylidene]-1,3-dihydro-indol-2-one and their in vitro antimicrobial activity. Res Chem Intermed. 2015;41:2953–9.
Winkelmann E,Raether W,Gebert U,Sinharay A, Chemotherapeutically active nitro compounds. 4. 5-Nitroimidazoles (part I). Arzneimittelforschung. 1977;27:2251–63.
Ang CW, Jarrad AM, Cooper MA, Blaskovich MAT. Nitroimidazoles: molecular fireworks that combat a broad spectrum of infectious diseases. J Med Chem. 2017;60:7636–57.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (Grant NO. 81602956 and 81473253), National Major Program of China during the 13th Five-Year Plan Period (Grant NO. 2018ZX09721001-001-001), and China Postdoctoral Science Foundation (Grant NO. 2016M590895). Thank Sichuan Provincial People’s Hospital for the supply of VRE B148 strains.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, Y., Ju, Y., Yang, Y. et al. Discovery of hybrids of indolin-2-one and nitroimidazole as potent inhibitors against drug-resistant bacteria. J Antibiot 71, 887–897 (2018). https://doi.org/10.1038/s41429-018-0076-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0076-5
This article is cited by
-
Design and synthesis of novel spirooxindole–indenoquinoxaline derivatives as novel tryptophanyl-tRNA synthetase inhibitors
Molecular Diversity (2020)
-
Synthesis and antibacterial activity of 3-substituted 1-(2-methyl-5-nitrophenyl)-5-oxopyrrolidine derivatives
Research on Chemical Intermediates (2019)