Abstract
We synthesized side-chain crystalline block copolymer that exhibit an adsorptive interaction with polyethylene (PE) using a monomer with a long alkane side chain and a hydrophilic monomer. By coating PE with a dilute solution of these block copolymers, the PE surface can easily be endowed with hydrophilicity in a uniform manner. This method does not require a special experimental apparatus or reagents and is more viable than conventional methods in terms of energy efficiency and cost, which means it has a lower environmental impact. Based on the measured contact angle on the modified PE surface, we also evaluated the effects of copolymer concentration in the coating solution and the degree of polymerization in the functional region of the copolymer. The results suggest that the hydrophilicity of the PE surface can be controlled by adjusting these parameters.
Similar content being viewed by others
Introduction
Polyethylene (PE) accounts for ~30% of the total of plastic production in the world [1]. It has been widely used in consumer products (such as pipes and packaging materials) and in industrial applications [2,3,4]. Even today, the use of PE continues to grow, primarily due to its qualities, such as water resistance, chemical resistance, and insulation characteristics, in addition to its low cost, light weight, and high workability. Newer applications of PE introduced in recent years include thermal conductivity materials and separators for lithium-ion batteries [5, 6]. However, due to its almost nonpolar surface and chemical stability, PE has low hydrophilicity, poor coating characteristics, and low adhesion. Therefore, the use of PE in applications that require hydrophilicity or adhesion is difficult.
There are extensive studies on expanding the uses of PE through functionalization. One typical traditional approach is creating composite materials such as laminates and polymer alloys, and many products using these methods have been on the market [7,8,9]. However, these methods could only achieve some of the intended properties due to lack of precision of the kneading technique, composition ratio, injection molding, etc.; therefore, special technology and devices are required for composite production. Recently, methods to modify only the surface of PE have been studied [10]. Since it is difficult to modify PE by chemical treatments due to its high chemical stability, most of these studies utilized the following physical treatments: UV irradiation, plasma treatment, corona treatment, and flame treatment [11,12,13,14]. These physical methods succeeded in imparting hydrophilicity, adhesion, and other properties by introducing functional groups such as hydroxyl and carboxyl groups onto the surface while maintaining the bulk properties of PE. However, these methods not only require a plasma generator or other sophisticated equipment but also have difficulties in achieving uniform modification and a long-lasting modification effect. It is necessary to increase the duration and intensity of irradiation to achieve uniform modification over the surface, and graft polymerization needs to be performed to maintain the effect for a long time. Because of the high energy consumption, cost, and environmental impact of these methods, there has been a growing need for new, simple, and effective modification techniques.
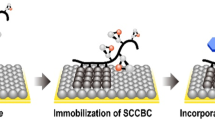
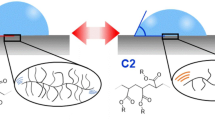
In our previous studies, a side-chain crystalline block copolymer (SCCBC) was synthesized from a monomer with a long alkane side chain (side-chain crystalline unit) and a functional (such as solvent affinity) monomer (functional unit) (Fig. 1). Then, it was used for modification of PE surface in a simple and uniform manner [15,16,17]. The results clearly show that as the molecular weight of the side-chain crystalline unit decreased (under ~3000 g mol−1), this SCCBC lost its modification ability [18,19,20]. Moreover, the PE particle dispersion system shows significant dispersant ability at very low concentration (~0.5 wt%) when the crystalline unit has a high-enough molecular weight. Figure 2 shows a schematic diagram of the adsorption mechanism of SCCBC onto PE. The crystal structure of the long alkane chain region of SCCBC is similar to that of PE, which appears via the formation of cocrystals, causing the strong adsorption of SCCBC onto the PE surface. As a result, the functional region covers the PE surface like a brush, which enables the addition of specific characteristics by using different functional monomers. Accordingly, it is possible to introduce hydrophilicity, adhesion, biocompatibility, dyeability, and other desired properties onto the PE film surface, which is difficult to achieve with surface modification. Figure 3 shows examples of typical contact angle measurements revealing the modification ability of SCCBC. Figure 3a shows the original non-modified PE film, which shows hydrophilicity with a water contact angle of 85°. In Fig. 3b, the examined film is modified with homo side-chain crystalline polymer by coating with 0.31 wt% solution. In this case, the hydrophobicity is decreased with a water contact angle of 101°. On the other hand, in the inset of Fig. 3c, a PE film that is modified with SCCBC by coating with SCCBC solution (0.31 wt%) shows an increase in hydrophilicity with a water contact angle of 56°. This result shows that SCCBC has high PE modification ability.
However, among practical modification processes, solution coating is the most useful and popular method. With the solution coating process, there are many factors that need to be considered to obtain stable and fine properties, such as block copolymer solution concentration and unit length and polymer molecular weight. In this study, we synthesized two SCCBCs with hydrophilic polyethylene glycol chains in the functional region. Then, the structure was determined by analytical methods, including gel permeation chromatography (GPC) and infrared (IR) spectroscopy. The hydrophilicity of the PE film coated with SCCBC was evaluated by measuring the contact angles. Additionally, the effect of the solution concentration and composition of SCCBC on the hydrophilicity of the coated PE was studied based on the contact angle measurement in combination with scanning electron microscopy (SEM) analysis.
Experimental
Materials
Hexadecyl acrylate (HDA, Tokyo Chemical Industry Co., Ltd. Tokyo, Japan) and di(ethylene glycol)ethyl ether acrylate (DEEA, Sigma-Aldrich Co., Llc., St. Louis, Mo, USA) were used for polymerization. BlocBuilder® MA (Arkema Inc., Colombes, France) was used as the radical initiator. Solvents such as butyl acetate and isopropyl alcohol (Wako Pure Chemical Industries, Ltd., Osaka, Japan) were used for reactions, re-precipitation, and modification.
Polymerization of SCCBC
The SCCBCs were synthesized by nitroxide-mediated polymerization (NMP), a living radical polymerization method that enables control of polymerization only by heating without external radical sources or metal catalysts. In the experiment, polymerization was initiated by heating a solution containing HDA and a radical initiator at 105 °C. The reaction was monitored by GPC, and consumption of HDA was confirmed 6 h later. After the reaction was complete, DEEA was added to the reaction solution, heated, and purified by re-precipitation. The degree of polymerization of SCCBC can be easily changed by varying the amount of the monomer. In the case of SCCBC1, 10.0 g (33.7 mmol) of HDA, 10.0 mL of butyl acetate, and 0.767 g (1.0 mmol) of BlocBuilder® MA were added to a nitrogen-purged separable flask. After 6 h, a solution of DEEA (6.07 g, 32.2 mmol) in butyl acetate (6.00 mL) that had been deaerated in advance was added to the flask. One day later, the contents of the flask were exposed to air and cooled rapidly to stop polymerization. In the case of SCCBC2, 5.04 g (16.9 mmol) of HDA, 5.0 mL of butyl acetate, and 0.381 g (0.5 mmol) of BlocBuilder® MA were added to a nitrogen-purged separable flask. After 6 h, a solution of DEEA (15.1 g, 80.1 mmol) in butyl acetate (15.00 mL) that had been deaerated in advance was added to the flask. The reactants were dissolved in isopropyl alcohol, allowed to undergo re-precipitation in water three times, and dried under vacuum.
Characterization of SCCBC
The molecular weight of the samples collected during reaction monitoring and the purified SCCBC were measured by GPC (HLC-8320GPC EcoSEC, Tosoh Corporation, Tokyo, Japan). FT-IR spectra were obtained with a spectrometer (Spectrum Two FT-IR spectrometer, PerkinElmer Inc., Waltham, MA, USA) to identify the chemical structure of SCCBC. The melting temperature of SCCBC was measured by differential scanning calorimetry (DSC; DSC8500, PerkinElmer Inc.). This measurement was performed in the order of warming, cooling, and warming in the temperature range of 0–50 °C and 50–0 °C at a rate of 5 °C min−1. The X-ray diffraction (XRD) patterns of the SCCBCs were also obtained.
Method of coating SCCBC on polyethylene
Solutions of SCCBC were prepared by adding varying amounts of SCCBC (0.16, 0.31, 0.63, 1.25, 2.50, and 5.00 wt%) in 5 g of butyl acetate. Using a #5 wire bar (11.43 µm) with a coater (TC-36PE, MitsuiElectric Co., Ltd.), 2 g of the solution was applied to a PE film (Petrosen®170, 5 cm × 5 cm, Tosoh Corporation). Subsequently, the solvent was removed by vacuum drying.
Evaluation of modification effect
The concentration of SCCBC on a modified PE surface was evaluated by FT-IR. The contact angle was measured (DropMaster DM-301, Kyowa Interface Science Co., Ltd.) to evaluate the hydrophilicity of the PE films modified with SCCBC solutions. The angles were measured five times for each film, and the mean value was calculated. The microstructures of the modified PE surfaces were observed by SEM (JSM-6060, JEOL Ltd.). Furthermore, after applying different concentrations of solutions onto the film, IR analysis was performed.
Results and discussion
Physical properties of SCCBC
Table 1 summarizes the GPC and DSC results of polymerized SCCBC1 and SCCBC2. SCCBC1 was obtained as a white solid, and the weight-average molecular weight (Mw) of HDA and DEEA was 3000 and 9000 g mol−1, respectively. We successfully synthesized SCCBC2 with Mw values of 4000 and 16,000 g mol−1 for HDA and DEEA, respectively. The polydispersity (Mw/Mn) of each SCCBC is also listed in Table 1. The polydispersity of SCCBC2 is greater than that of SCCBC1. The DSC results show that each SCCBC has a single melting point: 25 and 30 °C for SCCBC1 and SCCBC2, respectively.
The chemical structures of the synthesized SCCBCs were identified by FT-IR analysis. Figure 4 shows the spectra of the HDA and DEEA monomers and the synthesized SCCBCs. The spectra of the two monomers show a stretching vibration corresponding to C=C near 1650 cm−1 and another stretching vibration corresponding to C=O near 1750 cm−1. Furthermore, the spectrum of HDA has an absorption peak attributable to the stretching vibration of –CH at 2800–2900 cm−1, which is considered to originate from the long-chain alkyl group. On the other hand, the spectrum of DEEA has a characteristic peak derived from C–O–C at 1000–1200 cm−1. The spectra of the synthesized SCCBCs do not show any peaks corresponding to C=C double bonds. Instead, they only show the absorptions of the long-chain alkyl group and ether group characteristic of HDA and DEEA, indicating that the polymerization was successful.
Conformational analysis of SCCBC
Figure 5 shows XRD diffraction patterns of the original non-modified PE and SCCBCs. The pattern of the non-modified PE has peaks at 22 and 25°, which correspond to the (110) and (200) planes. The XRD patterns of SCCBCs show a crystallization peak at approximately the same position as that of (110) in the pattern of PE. This suggests that the side-chain crystalline regions of SCCBCs have a crystal plane spacing that is close to the (110) spacing of PE. The side-chain crystalline region of the SCCBC may form cocrystals on the PE surface, thereby allowing the adsorption of SCCBC. The results also indicate the possibility of making PE hydrophilic by utilizing the characteristics of the functional region of DEEA, which covers the PE surface like the bristles on a brush.
Characterization of modified PE surfaces
Figure 6 shows the FT-IR spectra after modification of PE by coating with SCCBC solution, which was conducted using a coater with SCCBC solutions at varying concentrations. The FT-IR data for the modified PE films show the SCCBC-derived absorption peak (stretching vibration corresponding to C = O near 1,750 cm−1) and the DEEA-derived absorption peak (C-O-C at 1,000–1,200 cm−1). Additionally, the peaks increased with increasing SCCBC concentrations compared with those of the non-modified PE film. Therefore, the amounts of SCCBC present on the PE surface can be tuned by changing its concentration in the coating solution.
Effect of the solution concentration and functional region length
Figure 7 shows photographs of contact angle measurements performed to evaluate the hydrophilicity of PE modified with SCCBC1 solutions at various concentrations. The contact angle of the original non-modified PE film was 85.4°, while that of PE modified with 0.16 wt% SCCBC1 solution was lowered to 70.1°. As the concentration of SCCBC solution increased, the contact angle tended to decrease, reaching 56.0° when modified with 0.31 wt% SCCBC1 solution. Hence, we confirmed that PE was successfully made hydrophilic by simple coating with SCCBC1 solution. Interestingly, at concentrations >0.31 wt%, the contact angle did not decrease further but remained about the same or increased slightly. For example, the contact angle at 5.00 wt% was 59.3°, which is significantly lower than that of non-modified PE but slightly higher than that modified with 0.31 wt% SCCBC1. Based on the above results, a solution concentration of 0.31 wt% was chosen as the optimum concentration to maximize the modification effect of SCCBC1 solution.
The effect of the solution concentration and length of the functional region on hydrophilicity was studied. We consider that the hydrophilicity of PE can be deliberately controlled by this modification technique by changing the solution concentration and length of the functional region of SCCBC. Figure 8 shows the contact angles on SCCBC1-modified PE films (a) and SCCBC2-modified PE films (b). From Fig. 8a, as mentioned previously, the concentration dependence of the contact angle shows a minimum value at 0.31 wt% and remains at almost same value up to the highest concentration (5.00 wt%). Accordingly, the contact angle was measured for PE modified by SCCBC2 with varying degrees of polymerization in the functional region while fixing the side-chain crystalline region. Figure 8b shows the contact angles on SCCBC2-modified PE. The minimum contact angle for SCCBC2 (DEEA (Mw = 16,000))-modified PE is 41.8° at 0.13 wt%, which is lower than that of SCCBC1 (DEEA (Mw = 9000)). This suggests that the amount of DEEA present on the surface increases as the degree of polymerization in the functional region increases, leading to an increase in hydrophilicity. Above the minimum value, the contact angle of the PE modified by SCCBC2 solution increased and reached 70.5° at 5.00 wt%. Interestingly, between SCCBC2 and SCCBC1, the slope on the right side of each plot (where the contact angle increases with concentration) was significantly steeper for the former polymer. These results indicate that the contact angle rapidly decreases at very dilute concentration and starts to increase above a certain value and that the ratio of this increase depends on the structure of SCCBC.
SEM observations
Finally, the surfaces of the PE modified with each SCCBC were analyzed by SEM. Figure 9a shows a surface SEM image of original non-modified PE. In Fig. 9b, the surface of PE modified with dilute SCCBC1 solution (0.31 wt%) is smoother than the non-modified surface, indicating that SCCBC1 was uniformly applied to the surface of PE through modification. In contrast, at high concentrations (5.00 wt%), in the images in Fig. 9c, there was an increased number of lumps that appear to be SCCBC1 accumulated on the PE surface. It is suspected that multiple layers of SCCBC1 were adsorbed at high concentrations, which disturbed the surface and led to a slight increase in contact angle. In the images in Fig. 9d, the surface of PE modified with dilute SCCBC2 solution (0.16 wt%) was similar to that in the case of SCCBC1: the surface became smoother after modification at low concentrations. At high concentrations (5.00 wt%), in the images in Fig. 9e, the lumpy surface disturbance with SCCBC2 became more significant than that with SCCBC1. This can be attributed to the larger molecular weight distribution in SCCBC2, which caused disordered lamination of SCCBC2 compared to the fine lamination achieved with SCCBC1. The disturbance on the surface at high concentrations is also more significant for SCCBC2 than for SCCBC1. As the concentration increases, the SCCBC layer becomes thicker by laminating the SCCBC molecules. When the polydispersity of SCCBC is small, the lamination may become regular, and the surface remains flat. On the other hand, when the polydispersity becomes large, the surface becomes rough.
It is well known that the wettability considerably depends on the morphology of the surface [21]. The different concentration dependence of SCCBC1 and SCCBC2 originates from both the length of the functional region and polydispersity of the molecular weight of SCCBC.
Conclusion
We developed a new effective surface modification technique of PE, namely, by surface coating with a dilute solution of SCCBC with the desired composition synthesized using an NMP method. This simple technique could produce a modified PE surface with a high degree of homogeneity. Furthermore, such coating only requires a simple experimental apparatus and mild reaction conditions. The change in surface hydrophilicity after modification can be controlled by the SCCBC concentration and the functional region length. In addition to hydrophilicity, in the future, other desirable properties (e.g., adhesion, dyeability, and cell affinity) can be imparted to PE surfaces by varying the structure of SCCBC and its solution composition.
References
The Japan plastics industry federation (JPIF). 2012. http://www.jpif.gr.jp/5topics/conts/world3_c.htm. Accessed 5 Dec 2017.
Ščetar M, Kurek M, Galić K. Trends in meat and meat products packaging – a review. Croat J Food Sci Technol. 2010;2:32–48.
Krishnaswamy RK. Analysis of ductile and brittle failures from creep rupture testing of high-density polyethylene (HDPE) pipes. Polymer. 2005;46:11664–72.
DesLauriers PJ, McDaniel MP, Rohlfing DC, Krishnaswamy RK, Secora SJ, Benham EA, Maeger PL, Wolfe AR, Sukhadia AM, Beaulieu BB. A comparative study of multimodal vs. bimodal polyethylene pipe resins for PE-100 applications. Polym Eng Sci. 2005;45:1203–13.
Shen S, Henry A, Tong J, Zheng R, Chen G. Polyethylene nanofibers with very high thermal conductivities. Nat Nanotechnol. 2010;5:251–5.
Yoneda H, Nishimura Y, Doi Y, Fukuda M, Kohno M. Development of microporous PE films to improve lithium ion batteries. Polym J. 2010;42:425–37.
Todd AD, McEneany RJ, Topolkaraev VA, Macosko CW, Hillmyer MA. Reactive compatibilization of poly(ethylene terephthalate) and high-density polyethylene using amino-telechelic polyethylene. Macromolecules. 2016;49:8988–94.
Su J, Zhang J. Comparison of rheological, mechanical, electrical properties of HDPE filled with BaTiO3 with different polar surface tension. Appl Surf Sci. 2016;388:531–8.
Ishihara K, Nishiuchi D, Watanabe J, Iwasaki Y. Polyethylene/phospholipid polymer alloy as an alternative to poly(vinylchloride)-based materials. Biomaterials. 2004;25:1115–22.
Brewis DM, Dahm RH. A review of electrochemical pretreatments of polymers. Int J Adhes Adhes. 2001;21:397–9.
Shina J, Liu X, Chikthimmah N, Lee YS. Polymer surface modification using UV treatment for attachment ofnatamycin and the potential applications for conventional food clingwrap (LDPE). Appl Surf Sci. 2016;386:276–84.
Zheng Y, Miao J, Zhang F, Cai C, Koh A, Simmons TJ, Mousa SA, Linhardt RJ. Surface modification of a polyethylene film for anticoagulant and antimicrobial catheter. React Funct Polym. 2016;100:142–50.
Nam K, Iwata T, Kimura T, Ikake H, Shimizu S, Masuzawa T, Kishida A. Adhesion between polymer surface modified by graft polymerization and tissue during surgery using an ultrasonically activated scalpel device. J Appl Polym Sci. 2014;131:40885.
Severini F, Landro LD, Galfetti L, Meda L, Ricca G, Zenere G. Flame surface modification of polyethtylene sheets. Macromol Symp. 2002;181:225–43.
Hirai S, Takeda M, Nitta N, Nakano R, Sekiguchi H, Yao S. Novel thermal rheological fluid with arterial embolic function, using side chain crystalline block copolymer. Kobunshi Ronbunshu. 2018;75:75–79.
Nakano R, Sekiguchi H, Yao S. Polyethylene surface modification by side chain crystalline block copolymer. Macromol Symp. 2015;349:44–50.
Sano Y, Nakano R, Sekiguchi H, Yao S. Solid electrolyte function of a polyethylene porous membrane filled with side-chain crystalline block co-polymer by using its crystalline supramolecular interaction. Int J Mater Sci Appl. 2014;3:399–3.
Yao S. Crystalline supramolecular interaction of side chain crystalline polymer and their future. Kobunshi Ronbunshu. 2016;73:139–46.
Yao S, Sakurai M, Sekiguchi H, Otsubo H, Uto T, Yamachika Y, Ishino W, Ichikawa S, Tatsumi D. Thermal rheological fluid properties of particle dispersion systems using side chain crystalline block copolymer III. Nihon Reoroji Gakkaishi. 2013;41:7–12.
Yao S, Ichikawa S. A novel dispersant for high content polyethylene particle dispersion. Nihon Reoroji Gakkaishi. 2011;39:181–82.
Takahara A, Kobayash M. Nature-inspired super hydrophilic and antifouling surface. J Surf Finish Soc Jpn. 2013;64:15–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Miho, Y., Hirai, S., Nakano, R. et al. Modification of polyethylene using side-chain crystalline block copolymer and evaluation of hydrophilicity. Polym J 50, 439–445 (2018). https://doi.org/10.1038/s41428-018-0031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-018-0031-0
This article is cited by
-
Selective acetylation of amorphous region of poly(vinyl alcohol) in supercritical carbon dioxide
Polymer Journal (2023)
-
Formation of a glyco-functionalized interface on polyethylene using a side-chain crystalline block copolymer with epoxide
Polymer Journal (2022)
-
A preliminary animal study of thermal rheology fluid as a new temperature-dependent liquid intravascular embolic material
Japanese Journal of Radiology (2022)