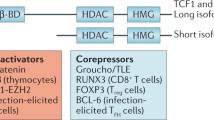
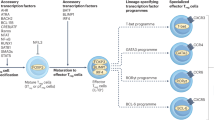
Abstract
Immune tolerance is a highly regulated state and involves diverse mechanisms. Central to the induction of tolerance is the targeted modulation of T-cell activities (both effector and regulatory), in which transcription factors play a significant role. The nuclear factor kappa-B (NF-κB) family is a family of transcription factors that not only are critically involved in diverse T-cell responses but also are regulated by many mechanisms to maintain tolerance and T-cell homeostasis. NF-κB, as a transcription factor, has been extensively studied in recent decades, and the molecular mechanisms that regulate NF-κB activities have been well documented. However, recent studies have revealed exciting new roles for NF-κB; in addition to its transcriptional activity, NF-κB can also activate diverse epigenetic mechanisms that mediate extensive chromatin remodeling of target genes to regulate T-cell activities. In this review article, we highlight recent discoveries and emerging opportunities in targeting NF-κB family members as well as their associated chromatin modifiers in the induction of immune tolerance and in the clinical treatment of immune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Van Parijs, L. & Abbas, A. K. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science 280, 243–248 (1998).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Sakaguchi, S. Naturally arising CD4 + regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Zhang, P. & Lu, Q. Genetic and epigenetic influences on the loss of tolerance in autoimmunity. Cell. Mol. Immunol. 15, 575–585 (2018).
Schwartz, R. H. Natural regulatory T cells and self-tolerance. Nat. Immunol. 6, 327–330 (2005).
Wood, K. J. & Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3, 199–210 (2003).
Malissen, B. & Bongrand, P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol. 33, 539–561 (2015).
Li, P., Spolski, R., Liao, W. & Leonard, W. J. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol. Rev. 261, 141–156 (2014).
Falvo, J. V., Jasenosky, L. D., Kruidenier, L. & Goldfeld, A. E. Epigenetic control of cytokine gene expression: regulation of the TNF/LT locus and T helper cell differentiation. Adv. Immunol. 118, 37–128 (2013).
Baldwin, A. S.Jr. TheNF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683 (1996).
Siebenlist, U., Franzoso, G. & Brown, K. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell. Biol. 10, 405–455 (1994).
Ghosh, S., May, M. J. & Kopp, E. B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
Ghosh, G., Wang, V. Y., Huang, D. B. & Fusco, A. NF-kappaB regulation: lessons from structures. Immunol. Rev. 246, 36–58 (2012).
Smale, S. T. Dimer-specific regulatory mechanisms within the NF-kappaB family of transcription factors. Immunol. Rev. 246, 193–204 (2012).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Perkins, N. D. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25, 6717–6730 (2006).
Hayden, M. S. & Ghosh, S. Shared principles in NF-kappaB signaling. Cell 132, 344–362 (2008).
Pires, B. R. B., Silva, R., Ferreira, G. M. & Abdelhay, E. NF-kappaB: Two sides of the same coin. Genes (Basel) 9,1–23 (2018).
Kanarek, N. & Ben-Neriah, Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 246, 77–94 (2012).
Chen, J. & Chen, Z. J. Regulation of NF-kappaB by ubiquitination. Curr. Opin. Immunol. 25, 4–12 (2013).
Sun, S. C. The noncanonical NF-kappaB pathway. Immunol. Rev. 246, 125–140 (2012).
Sun, S. C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Razani, B., Reichardt, A. D. & Cheng, G. Non-canonical NF-kappaB signaling activation and regulation: principles and perspectives. Immunol. Rev. 244, 44–54 (2011).
Sun, S. C. Non-canonical NF-kappaB signaling pathway. Cell Res. 21, 71–85 (2011).
Oeckinghaus, A., Hayden, M. S. & Ghosh, S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 12, 695–708 (2011).
Israel, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2, a000158 (2010).
Liu, F., Xia, Y., Parker, A. S. & Verma, I. M. IKKbiology. Immunol. Rev. 246, 239–253 (2012).
Xiao, G., Fong, A. & Sun, S. C. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 279, 30099–30105 (2004).
Yu, J. et al. T cell-intrinsic function of the noncanonical NF-kappaB pathway in the regulation of GM-CSF expression and experimental autoimmune encephalomyelitis pathogenesis. J. Immunol. 193, 422–430 (2014).
Lo, J. C. et al. Coordination between NF-kappaB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood 107, 1048–1055 (2006).
Hoffmann, A., Leung, T. H. & Baltimore, D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 22, 5530–5539 (2003).
Basak, S., Shih, V. F. & Hoffmann, A. Generation and activation of multiple dimeric transcription factors within the NF-kappaB signaling system. Mol. Cell. Biol. 28, 3139–3150 (2008).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430, 694–699 (2004).
Shembade, N. & Harhaj, E. W. Regulation of NF-kappaB signaling by the A20 deubiquitinase. Cell. Mol. Immunol. 9, 123–130 (2012).
Lu, T. T. et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 38, 896–905 (2013).
Beyaert, R., Heyninck, K. & Van Huffel, S. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-kappa B-dependent gene expression and apoptosis. Biochem. Pharmacol. 60, 1143–1151 (2000).
Duwel, M. et al. A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains. J. Immunol. 182, 7718–7728 (2009).
Lee, E. G. et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Catrysse, L., Vereecke, L., Beyaert, R. & van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 35, 22–31 (2014).
Iwai, K. & Tokunaga, F. Linear polyubiquitination: a new regulator of NF-kappaB activation. EMBO Rep. 10, 706–713 (2009).
Lork, M., Verhelst, K. & Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 24, 1172–1183 (2017).
Lich, J. D. et al. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J. Immunol. 178, 1256–1260 (2007).
Allen, I. C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity 36, 742–754 (2012).
Hu, H. et al. OTUD7B controls non-canonical NF-kappaB activation through deubiquitination of TRAF3. Nature 494, 371–374 (2013).
Fusco, A. J. et al. The NF-kappaB subunit RelB controls p100 processing by competing with the kinases NIK and IKK1 for binding to p100. Sci. Signal. 9, ra96 (2016).
Maminska, A. et al. ESCRT proteins restrict constitutive NF-kappaB signaling by trafficking cytokine receptors. Sci. Signal. 9, ra8 (2016).
Ma, X., Becker Buscaglia, L. E., Barker, J. R. & Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 3, 159–166 (2011).
Mao, X., Su, Z. & Mookhtiar, A. K. Long non-coding RNA: a versatile regulator of the nuclear factor-kappaB signalling circuit. Immunology 150, 379–388 (2017).
Dawson, M. A. The cancer epigenome: Concepts, challenges, and therapeutic opportunities. Science 355, 1147–1152 (2017).
Lim, P. S., Li, J., Holloway, A. F. & Rao, S. Epigenetic regulation of inducible gene expression in the immune system. Immunology 139, 285–293 (2013).
Torres, I. O. & Fujimori, D. G. Functional coupling between writers, erasers and readers of histone and DNA methylation. Curr. Opin. Struct. Biol. 35, 68–75 (2015).
Tessarz, P. & Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 15, 703–708 (2014).
Yang, J., Tian, B. & Brasier, A. R. Targeting Chromatin Remodeling in Inflammation and Fibrosis. Adv. Protein Chem. Struct. Biol. 107, 1–36 (2017).
Xiao, X. et al. The costimulatory receptor OX40 inhibits interleukin-17 expression through activation of repressive chromatin remodeling pathways. Immunity 44, 1271–1283 (2016).
Xiao, X. et al. GITR subverts Foxp3( + ) Tregs to boost Th9 immunity through regulation of histone acetylation. Nat. Commun. 6, 8266 (2015).
Zhang, X. et al. OX40 costimulation inhibits Foxp3 expression and treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 24, 607–618 (2018).
Zhong, H., May, M. J., Jimi, E. & Ghosh, S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell 9, 625–636 (2002).
Yeung, F. et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Xiao, X. et al. Guidance of super-enhancers in regulation of IL-9 induction and airway inflammation. J. Exp. Med. 215, 559–574 (2018).
Salomoni, P. & Khelifi, A. F. Daxx: death or survival protein? Trends Cell Biol. 16, 97–104 (2006).
Puto, L. A. & Reed, J. C. Daxx represses RelB target promoters via DNA methyltransferase recruitment and DNA hypermethylation. Genes Dev. 22, 998–1010 (2008).
Liu, Y. et al. Phosphorylation of RelA/p65 promotes DNMT-1 recruitment to chromatin and represses transcription of the tumor metastasis suppressor gene BRMS1. Oncogene 31, 1143–1154 (2012).
DiDonato, J. A., Mercurio, F. & Karin, M. NF-kappaB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 (2012).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Loven, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Donati, B., Lorenzini, E. & Ciarrocchi, A. BRD4 and Cancer: going beyond transcriptional regulation. Mol. Cancer 17, 164 (2018).
Brown, J. D. et al. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56, 219–231 (2014).
Betancur, P. A. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 8, 14802 (2017).
Lanzillotta, A. et al. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol. Dis. 49, 177–189 (2013).
Schiaffino, L. et al. Acetylation state of RelA modulated by epigenetic drugs prolongs survival and induces a neuroprotective effect on ALS murine model. Sci. Rep. 8, 12875 (2018).
Brasier, A. R. et al. RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J. Virol. 85, 11752–11769 (2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Xiao, X., Arnold, P.R. et al. Transcriptional and epigenetic regulation of immune tolerance: roles of the NF-κB family members. Cell Mol Immunol 16, 315–323 (2019). https://doi.org/10.1038/s41423-019-0202-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0202-8
Key words
This article is cited by
-
Bilirubin Induces A1-Like Reactivity of Astrocyte
Neurochemical Research (2023)
-
The transcription factor RelB restrains group 2 innate lymphoid cells and type 2 immune pathology in vivo
Cellular & Molecular Immunology (2021)