Abstract
Periodontal ligament stem cells (PDLSCs) possess great potential for clinical treatment of immune diseases due to their extensive immunomodulatory properties. However, the underlying mechanisms that govern the immunomodulatory properties of mesenchymal stem cells (MSCs) are still not fully elucidated. Here, we show that member of the Ten-eleven translocation (Tet) family, a group of DNA demethylases, are capable of regulating PDLSC immunomodulatory functions. Tet1 and Tet2 deficiency enhance PDLSC-induced T cell apoptosis and ameliorate the disease phenotype in colitis mice. Mechanistically, we found that downregulation of Tet1 and Tet2 leads to hypermethylation of DKK-1 promoter, leading to the activation of WNT signaling pathway and therefore promoting Fas ligand (FasL) expression, which results in elevated immunomodulatory capacity of PDLSCs. These results reveal a previously unrecognized role of Tet1 and Tet2 in regulating immunomodulation of PDLSCs. This Tet/DKK-1/FasL cascade may serve as a promising target for enhancing PDLSC-based immune therapy.
Similar content being viewed by others
Introduction
The Ten-eleven translocation (Tet) family is a group of recently identified demethylases capable of modifying DNA by hydroxylating 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC)1,2,3. This discovery revealed a novel mechanism by which Tet enzymes regulate DNA demethylation. Three Tet family members (Tet1, Tet2, and Tet3) show distinct expression patterns depending on cell or tissue type and developmental stage4,5. For instance, Tet1 is highly expressed in mouse embryonic stem cells (ESCs); upon ESC differentiation, its expression level is significantly decreased. However, some terminally differentiated cells, such as Purkinje neurons, also show upregulated Tet1 expression2,6,7. Emerging evidences indicate that Tet-mediated DNA demethylation contributes to normal growth and developmental processes as well as disease development. Alteration of Tet expression has been linked to tumorigenesis, neural disorders, and T cell-related immune disorders8. Our previous studies showed that Tet1 and Tet2 play a crucial role in maintaining bone marrow mesenchymal stem cell (BMMSC) functions and bone homeostasis. Tet1 and Tet2 depletion resulted in osteopenia and impairment of BMMSC differentiation9, suggesting that the Tet family is able to modulate MSC biological function. Moreover, we have found that Tet1 and Tet2 help to maintain immune homeostasis via regulating regulatory T (Treg) cell function10. Whether Tet1/Tet2-mediated DNA demethylation modulates MSC-mediated immunomodulation remains unclear.
Periodontal ligament stem cells (PDLSCs), isolated from periodontal ligament tissue, possess unique immunomodulatory capacity11. PDLSCs interact with different immune cells, for example, suppressing T cell proliferation12 and upregulating Tregs13. After transplantation in a periodontitis minipig model, PDLSCs were able to modulate the local immune microenvironment and result in periodontal tissue regeneration14. Therefore, it is possible to use PDLSCs to treat inflammatory diseases14,15, such as periodontal disease.
In the present study, we demonstrated that Tet1/Tet2-mediated DNA demethylation is capable of regulating the immunomodulatory function of PDLSCs. Tet1 and Tet2 deficiency enhances PDLSC-induced T cell apoptosis and ameliorates the disease phenotype in colitis mice.
Results
Human PDLSCs express Tet1 and Tet2
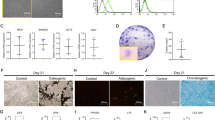
We found that human PDLSCs (hPDLSCs) isolated from human periodontal ligament expressed elevated levels of Tet1 and Tet2, as assessed by Western blotting and real-time polymerase chain reaction (qPCR), when compared to human BMMSCs (hBMMSCs) (Fig. 1a, b). Immunostaining showed that MSC marker CD146 was co-expressed with Tet1 and Tet2 in hPDLSCs (Fig. 1c). These data indicate that PDLSCs express certain levels of Tet1 and Tet2.
a, b Both human bone marrow mesenchymal stem cells (hBMMSCs) and hPDLSCs expressed Tet1 and Tet2, as assessed by Western blotting (a) and qPCR (b). c Immunocytofluorescent staining showed that CD146-positive hPDLSCs express Tet1 and Tet2. Scale bar, 50 µm. ***P < 0.001, **p < 0.01; p values were calculated using two-tailed Student’s t test (mean ± SD)
Tet1 and Tet2 regulate PDLSC-mediated immunomodulation
To investigate whether Tet1 and Tet2 mediate DNA demethylation that may regulate the multi-lineage differentiation and immunomodulation capacities of PDLSCs, we knocked down Tet1 and Tet2 expression in PDLSCs by using small interfering RNAs (siRNAs) (Fig. 2a). Bromodeoxyuridine (BrdU)-labeling assays showed that knocking down Tet1 and Tet2 in PDLSCs led to upregulated proliferation when compared with the control group (Fig. 2b). Flow cytometric analysis showed that MSC surface markers, including CD105 and CD73, were significantly elevated in Tet1/Tet2 siRNA-treated PDLSCs compared with control PDLSCs, but not CD90. The hematopoietic lineage markers, CD34 and CD45, were absent in Tet1/2 siRNA-treated PDLSCs, similar to the observations of control PDLSCs (Fig. 2c). Furthermore, we cultured PDLSCs and Tet1/Tet2 siRNA-treated PDLSCs under osteogenic and adipogenic differentiation condition and found that Tet1 and Tet2 deficiency led to significantly decreased osteogenic (Fig. S1a, b) and adipogenic (Fig. S1c, d) differentiation potential when compared to the control PDLSCs.
a Western blot analyzed the efficiency of Tet1 and Tet2 small interfering RNA (siRNA) knockdown in hPDLSCs. b BrdU labeling assay was performed to show elevated proliferation rates of Tet1 and Tet2 siRNA-treated hPDLSCs. Scale bar, 50 µm. c Flow cytometry was used to analyze the expression of CD105, CD90, CD73, CD34, and CD45 in control and Tet1 and Tet2 siRNA-treated hPDLSCs. d, e In vitro coculture showed a significantly increased capacity of Tet1 and Tet2 siRNA-treated hPDLSCs to induce T cell apoptosis (AnnexinV+7AAD+) of T cells. ***P < 0.001, **p < 0.01, and *p < 0.05; p values were calculated using two-tailed Student’s t test (mean ± SD)
Next, to evaluate the immunomodulatory properties of PDLSCs, we co-cultured PDLSCs with T cells and found that PDLSCs were capable of inducing T cell apoptosis16. Moreover, Tet1/Tet2 siRNA-treated PDLSCs had a significantly elevated capacity to induce AnnexinV+7AAD+ double-positive T cell apoptosis, when compared to the control PDLSCs (Fig. 2d, e). These results indicate that the inhibition of Tet1 and Tet2 promotes PDLSCs’ immunomodulatory capacity by inducing T cell apoptosis.
Inhibition of Tet1 and Tet2 enhances therapeutic effect of PDLSCs in treating colitis
The ability of MSCs to modulate immune response is one of their most important characteristics17,18. To further assess the role of Tet1 and Tet2 in immunomodulation by PDLSCs, we compared the immunotherapeutic effects of control and Tet1/Tet2 siRNA-treated PDLSCs in experimental colitis mice. C57BL/6J mice were orally administered 3% dextran sodium sulfate (DSS) for 10 days to establish acute colitis. On day 3, colitis mice were treated with Tet1/Tet2 siRNA-treated PDLSCs or control PDLSCs by systemic transplantation through tail vein, followed by sacrifice of the mice on day 10 to collect samples for evaluation (Fig. 3a).
a Schema showing PDLSC transplantation for treating colitis mice. b–e Knockdown of Tet1 and Tet2 by siRNA treatment elevated the immunomodulatory capacity of PDLSCs, as assessed by amelioration of the reduced body weight (b), a decreased disease activity index (DAI) (c), and alleviation of the colitis histologic activity index (HAI). Scale bar in d, 100 µm. f Flow cytometry analysis showed that the Treg level significantly decreased in colitis mice compared to control littermates. After PDLSC treatment, the Treg level was significantly elevated, and the Tet1 and Tet2 siRNA-treated PDLSC group showed a higher Treg level than the group with control PDLSCs. ***P < 0.001, **p < 0.01, *p < 0.05; p values were calculated using two-tailed Student’s t test (mean ± SD)
Consistent with previous reports19, DSS-induced colitis mice lost weight at a sustained rate and exhibited bloody diarrhea/loose feces, which were characterized by an overall evaluation of their condition using the established disease activity index (DAI). Infusion of either Tet1/Tet2 siRNA-treated PDLSCs or control PDLSCs partially restored the reduced body weight of the colitis mice and decreased their DAI scores. Furthermore, Tet1/Tet2 siRNA pretreatment was able to enhance the ability of PDLSCs to restore the reduced body weight (Fig. 3b) and decreased the DAI scores (Fig. 3c). Histologically, localized inflammatory cell infiltration, epithelial ulceration, and impairment of crypt architecture were observed in the intestines of DSS-induced colitis mice. Systemic infusion of either Tet1/Tet2 siRNA-treated PDLSCs or control PDLSCs could recover impaired intestinal structure. However, Tet1/Tet2 siRNA-treated PDLSCs demonstrated a superior ability to eliminate the inflammatory cells and recover the epithelial structure, as assessed by the histologic activity index (HAI) (Fig. 3d, e). Moreover, decreased number of CD4+CD25+Foxp3+ Tregs were observed in the mice with colitis at day 10 post-DSS induction (Fig. 3f). Infusion of control PDLSCs elevated the number of Treg cells, and Tet1/Tet2 siRNA pretreatment enhanced this effect. Taken together, these experimental data indicate that Tet1 and Tet2 inhibition results in an elevated capacity for PDLSC-mediated immunomodulation.
Tet1 and Tet2 regulate FasL expression through Wnt/β-catenin pathway in PDLSCs
To explore the underlying mechanism of how Tet1 and Tet2 regulate immunomodulation of PDLSCs, we examined the levels of FasL, a key member of the apoptosis pathway, in PDLSCs. It has been reported that FasL is related to MSCs’ immunomodulatory ability, as a previous study demonstrated that FasL in MSCs induced T cell apoptosis and immune tolerance20. Thus, in order to confirm that PDLSCs express FasL, we used double immunostaining and found that CD146-positive PDLSCs expressed FasL (Fig. 4a). To investigate whether Tet1 and Tet2 modulate the FasL expression level, Western blot and qPCR analysis were used, and it revealed that FasL was significantly elevated in Tet1/Tet2 siRNA-treated PDLSCs (Fig. 4b, c), indicating that the inhibition of Tet1 and Tet2 upregulates FasL expression and results in an elevation of MSC-based immunomodulatory ability. Next, to evaluate whether FasL is a direct target of Tet1 and Tet2, we used the Methprimer software to detect the promoter of FasL and found that the FasL promoter lacks CpG island, which indicates that Tet1 and Tet2 may not prefer to bind to the promoter of FasL21,22. Thus, we continued searching for potential molecules that may connect Tet with FasL. Since β-catenin could serve as a transcriptional factor to regulate FasL expression23,24, we next examined the Wnt/β-catenin expression levels in Tet1/Tet2 siRNA-treated PDLSCs. Western blotting showed that the expression level of active β-catenin (non-phosphorylated) was indeed significantly elevated, although the total level of β-catenin was not significantly elevated in Tet1/Tet2 siRNA-treated PDLSCs (Fig. 4c, d). The expression levels of active β-catenin and FasL were significantly decreased after the treatment of β-catenin inhibitor (FH535) (Fig. 4e, f). When co-cultured with T cells, Tet1/Tet2 siRNA-treated PDLSCs gained an elevated capacity to induce AnnexinV+7AAD+ double-positive T cell apoptosis when compared to the untreated group, but the elevated induction of apoptosis could be abrogated by β-catenin inhibitor (FH535) treatment (Fig. 4g). These results suggest that Tet1 and Tet2 act as upstream regulators of Wnt/β-catenin signaling to modulate FasL expression, which in turn regulates the PDLSC-mediated immunomodulatory capacity.
a Immunocytofluorescent staining showed that CD146-positive hPDLSCs express FASL. Scale bar, 50 µm. b qPCR analysis showed the significantly increased expression level of Fasl in Tet1 and Tet2 siRNA-treated hPDLSCs compared to control hPDLSCs. c, d Western blot analysis showed increased levels of FASL and active β-catenin in Tet1 and Tet2 siRNA-treated hPDLSCs compared to the control hPDLSCs. e, f β-Catenin inhibitor (FH535) treatment decreased the levels of FASL and active β-catenin in Tet1 and Tet2 siRNA-treated hPDLSCs. g In vitro coculture showed β-catenin inhibitor (FH535) treatment decreased the capacity of Tet1 and Tet2 siRNA-treated hPDLSCs to induce apoptosis (AnnexinV+7AAD+) of T cells. ***P < 0.001, **p < 0.01, *p < 0.05; p values were calculated using two-tailed Student’s t test (mean ± SD)
Furthermore, besides FasL, many other target genes can be regulated by β-catenin. Therefore, we analyzed Notch and tumor growth factor-β signaling-related genes, which have been identified as the downstream of WNT/β-catenin pathway25,26. Western blot analysis showed that the expression level of P-smad3 was decreased, while Notch1 and Notch2 showed an elevated expression in Tet1/Tet2 siRNA-treated PDLSCs compared with the control group (Fig. S2). These results indicated that Tet might be able to regulate other WNT-associated genes.
Tet1 and Tet2 serve as demethylases on DKK-1 promoter to regulate FasL expression in PDLSCs
To investigate how Tet1/Tet2 regulate Wnt/β-catenin signaling, we examined the level of DKK-1, an upstream inhibitor of the WNT pathway27, and found that DKK-1 was downregulated in Tet1/Tet2 siRNA-treated PDLSCs. Immunofluorescence staining showed co-localization of DKK-1 with MSC surface marker CD146 (Fig. 5a). Western blotting (Fig. 5b) and qPCR (Fig. 5c) showed that the expression level of DKK-1 was significantly decreased in Tet1/Tet2 siRNA-treated PDLSCs compared to the control group. As previous studies indicated that Tet1 and 5-hmC prefer to co-localize at CpG-rich locus in the promoter transcriptional start sites21,22, we examined the promoter region of DKK-1 (DKK-1 −1500 to 0, Homo sapiens chromosome 10, GRCh38 52,312,781 to 52,314,281) by using the Methprimer software; three CpG islands (DKK-1 −1048 to −943, −915 to −804, −286 to −176) were detected (Fig. 5d). To demonstrate if Tet1 and Tet2 are able to regulate DKK-1 directly, chromatin immunoprecipitation-qPCR (ChIP-qPCR) analysis were used to verify that Tet1 and Tet2 were able to bind to the CpG islands of the DKK-1 promoter (Fig. 5e). We next examined whether inhibition of Tet1 and Tet2 affected the enrichment of 5-hmC level at the DKK-1 promoter. Hydroxymethylated DNA immunoprecipitation-qPCR (hMeDIP-qPCR) analysis showed that Tet1/Tet2 siRNA-treated PDLSCs displayed a significantly decreased 5-hmC level compared to control PDLSCs on the three CpG island sites of the DKK-1 promoter (Fig. 5f). Oxidative bisulfite-sequencing (OxBS) sequencing analysis also showed an elevated methylation status in the promoter of DKK-1 locus in Tet1/Tet2 siRNA-treated PDLSCs compared to control PDLSCs (Fig. 5g). These results demonstrated that Tet1 and Tet2 are able to directly regulate DKK-1 expression through DNA demethylation. Next, we added DKK-1 to Tet1/Tet2 siRNA-treated PDLSCs and found that exogenous DKK-1 could block the increased expression of active β-catenin and FasL that was induced by Tet1/Tet2 siRNA treatment (Fig. 5h). When co-cultured with T cells, Tet1/Tet2 siRNA-treated PDLSCs gained an elevated capacity to induce AnnexinV+7AAD+ double-positive T cell apoptosis when compared to the untreated group, but that effect could be abrogated by DKK-1 treatment (Fig. 5i). Taken together, these findings indicate that the inhibition of Tet1 and Tet2 could promote PDLSC-mediated immunomodulation ability through regulating DKK-1 demethylation (Fig. 6).
a Immunocytofluorescent staining showed that CD146-positive hPDLSCs express DKK-1. Scale bar, 50 µm. b Western blot analysis showed decreased levels of DKK-1 in Tet1 and Tet2 siRNA-treated hPDLSCs compared to the control hPDLSCs. c qPCR analysis showed significantly decreased expression of DKK-1 in Tet1 and Tet2 siRNA-treated hPDLSCs compared to the control hPDLSCs. d The CpG island of DKK-1. e Tet1 and Tet2 binding on the promoter of DKK-1 in hPDLSCs, as assessed by ChIP-qPCR. IgG was used as a control. f Enrichment of 5-hmC in the DKK-1 promoter decreased in Tet1 and Tet2 siRNA-treated hPDLSCs. g OxBS-sequencing analysis showed that Tet1 and Tet2 siRNA-treated hPDLSCs displayed elevated methylation in the promoter of DKK-1 locus compared to control hPDLSCs. Empty dots indicate unmethylated GpGs; black dots indicate methylated CpGs. h DKK-1 treatment increased the level of DKK-1 and decreased the expression levels of FASL and active β-catenin in Tet1 and Tet2 siRNA-treated hPDLSCs, assessed by Western blot. i In vitro coculture showed that DKK-1 treatment decreases the capacity of Tet1 and Tet2 siRNA-treated hPDLSCs to induce apoptosis (AnnexinV+7AAD+) of T cells. ***P < 0.001, **p < 0.01; p values calculated using two-tailed Student’s t test (mean ± SD)
Discussion
Tet-mediated conversion of 5-mC to 5-hmC has been proposed as the initial step in active DNA demethylation, which plays an important role in maintaining tissue homeostasis10,28,29. Inhibition of Tet leads to deficiency of Tregs28 and myeloid invariant natural killer T cells, as well as B cell malignancy30,31,32. Our previous study demonstrated that Tet1 and Tet2 are required to maintain bone homeostasis via regulating the osteogenic differentiation of BMMSCs9,33. Here, we showed that inhibition of Tet1 and Tet2 led to decreased osteogenic and adipogenic differentiation of PDLSCs (Fig. S1). Moreover, we showed that Tet1 and Tet2 deficiency promoted the proliferation of hPDLSCs (Fig. 2b), in agreement with our previous finding in mouse BMMSCs9. However, based on studies on umbilical cord (UC)-derived MSCs, loss of Tet1/2 can inhibit the ability of MSCs to replicate34, which implies that different effects of Tet1/2 on MSC proliferation may be regulated by specific molecules9,34,35.
MSCs possess great potential for treating immune diseases due to their extensive immunomodulatory properties16,20. Whether Tet participates in regulating MSC immunomodulation has remained unknown. In the present study, we demonstrated that inhibition of Tet1 and Tet2 promoted PDLSC-induced T cell apoptosis (Fig. 2d, e). Importantly, when PDLSCs were systematically infused into DSS-induced colitis mice, Tet1/Tet2-downregulated PDLSCs showed significantly increased therapeutic effects (Fig. 3). These results may provide a promising method to promote MSC-mediated therapeutic efficacy.
The multi-potent and immunomodulatory properties of MSCs make them a valuable cell source for regeneration and immune therapy36,37. The key issue in MSC biology is to illustrate the underlying mechanisms that control MSCs’ multiple distinct functions. In the present study, we demonstrated that Tet deficiency led to inhibition of PDLSC differentiation toward osteogenic and adipogenic lineages (Fig. S1), similar to the observations made concerning BMMSCs in our previous report9. Interestingly, we also found that inhibition of Tet1 and Tet2 could elevate PDLSC immunomodulatory potential (Figs. 2 and 3), which indicates that Tet1 and Tet2 might serve as switch genes to control MSC differentiation and immunomodulation. Downregulation of Tet1 and Tet2 resulted in differentiation deficiency as well as upregulation of immunomodulation ability in PDLSCs. However, based on our current understanding, more investigation is required to further illustrate the mechanisms of how Tet regulates MSCs’ distinct functions in differentiation and immunomodulation.
Mechanistically, we identified a novel role of Tet1 and Tet2 in binding directly to the DKK-1 promoters, and further identified that they act as an upstream regulator of Wnt/β-catenin pathway to modulate FasL expression (Fig. 6). Previous studies demonstrated that Wnt pathway inhibitor DKKs could be inactivated by DNA methylation in cancer cells to sustain tumor development38,39, which can be reversed by demethylation activity on the DKK promoter region40. In the present work, we reported that Tet1 and Tet2 bind to the promoter of DKK-1 to maintain its hypomethylation in PDLSCs. It is known that MSCs are able to induce T cell apoptosis via Fas/FasL pathway, resulting in upregulation of Tregs and immune tolerance20. β-Catenin could directly bind to the FasL promoter to drive gene expression at the transcriptional level23. Based on our findings, downregulation of Tet1 and Tet2 led to repression of DKK-1 by DNA methylation modification, which results in activation of Wnt pathway and further promotes FasL expression in PDLSCs to enhance their immunomodulatory ability. This study provided evidence to link Tet1/Tet2, which are DNA demethylases, to MSC-mediated immunomodulation. It has been reported that Tet is able to modulate Foxp3 demethylation in T cells to affect immune homeostasis10 and could directly target the promoters of microRNAs in cancer cells41. Except Fas/FasL pathway, we showed that the expression of P-smad3, Notch1, and Notch2 were altered after siRNA knockdown; however, whether PDLSC-mediated immunomodulation could be modulated by other molecules needs further investigation to illustrate the detailed mechanism.
In summary, we demonstrated that Tet1 and Tet2 participate in the regulation of MSC-mediated immunomodulation. Downregulation of Tet1 and Tet2 in PDLSCs improves their immunomodulatory functions by inhibiting DKK-1 expression through hypermethylation. Tet1 and Tet2 knockdown pretreatment in PDLSCs may be a promising approach to enhance MSC-mediated immune therapeutic effects.
Materials and Methods
Antibodies
Tet1 (ab191698), Tet2 (ab94580), and CD146 (ab24577) antibodies were purchased from Abcam (Cambridge, MA, USA). Phospho-β-catenin (Ser552) and β-catenin (15B8) antibodies were purchased from Invitrogen (Carlsbad, CA, USA). FASL (SC-33716) and DKK-1 (SC-374574) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). β-Actin antibody (A1978) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-CD4-PerCP (550765) and anti-CD25-APC (561048) were purchased from BD Biosciences (San Jose, CA, USA). Anti-CD3 (100208) and anti-CD28 (102112) were purchased from BioLegend (SanDiego, CA, USA)
Isolation and culture of hPDLSCs
Periodontal ligament tissues were obtained from healthy patients (18–25 years old) without any history of periodontal disease who were underdoing orthodontic extraction. The protocol of PDLSC isolation and cultivation was followed according to a previous publication by Seo et al.42; the procedure was approved by the Ethical Guidelines of Peking University (PKUSSIRB-201311103). Passage 3 PDLSCs were used in all experiments.
siRNA and chemical treatment
For siRNA transfection, PDLSCs were cultured under reduced serum medium (Opti-MEM, Gibco), and Tet1 siRNA (sc-90457), Tet2 siRNA (sc-88934), or control vehicle siRNA (sc-37007) (Santa Cruz Biotechnology) were treated with Lipofectamine reagent (Invitrogen), according to the manufacturer’s instructions. For chemical reagent treatments, serum-starved PDLSCs were treated with 10 μM Wnt/β-catenin inhibitor (FH535; Cayman Chemical), or human DKK-1 (PeproTech). Treated cells were collected for further experiments.
Western blotting
Mammalian protein extraction reagent (Thermo, Rockford, IL, USA) was used to lyse total protein. The protein was applied and separated on 4 to 12% NuPAGE gel (Invitrogen), which were then transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% nonfat dry milk and 0.1% Tween-20 for 1 h, which were then incubated with the primary antibodies at 4 °C overnight. We used horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology; 1:10,000) to treat the membranes for 1 h. By using Super Signal West Pico Chemiluminescent Substrate (Thermo) and BioMax film (Kodak, USA), the immuno-reactive proteins were detected.
Real-time PCR
Total RNA was isolated using miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. SuperScript III Reverse Transcriptase (RT) Kit (Invitrogen) was used to prepare the complementary DNA. qPCR was performed using SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and gene-specific primers. Detection was performed on a CFX96 Real-Time PCR System (Bio-Rad).
Immunofluorescent staining
PDLSCs (1 × 103/well) were cultured in chamber slides (Nunc, Rochester, NY, USA) and then fixed with 4% paraformaldehyde (PFA). The cells were incubated with primary antibodies at 4 °C overnight, and then treated with Alexa Fluor 568 or Alexa Fluor 488-conjugated secondary antibody (1:200, Invitrogen) for 1 h. Finally, slides were mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA).
T lymphocytes apoptosis assay
A total of 0.2 × 106 PDLSCs (with or without Tet1/2 siRNA treatment) were seeded on a 24-well culture plate (Corning) containing Dulbecco’s modified Eagle’s medium (Lonza, Basel, Switzerland) with 10% heat-inactivated fetal bovine serum, 10 mM HEPES, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate (Sigma-Aldrich), 1% non-essential amino acid (Cambrex, East Rutherford, NY, USA), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Activated T cells (1 × 106/well), which were isolated from mouse spleen, were pre-stimulated with plate-bound anti-CD3ε and anti-CD28 (2 μg/ml each; BD Bioscience) for 2 days, and then loaded directly on PDLSCs. After 24 h of coculture, CD3 antibody and AnnexinV Apoptosis Detection Kit I (BD Bioscience) were used to detect apoptotic T cells using a FACS flow cytometer.
Cell proliferation assay
PDLSCs were stained with BrdU antibody (1:200, Invitrogen) after being incubated with BrdU solution (1:100, Invitrogen) for 12 h, and then Alexa Fluor 568-conjugated secondary antibody were used to stain the cells for 1 h at room temperature. Finally, slides were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories). The percentage of BrdU-positive cell number in total cell number was calculated. Three independent samples of each experimental group were used for BrdU assay and BrdU-positive and total cell numbers were counted in 10 images per subject.
DSS-induced mouse colitis and treatment with PDLSCs
Female C57BL/6J (JAX #000664) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Acute colitis was induced in 8-week-old C57BL/6J mice with 3% (w/v) DSS (MP Biochemicals) in the drinking water for 10 days43. A total of 2.0 × 106 untreated PDLSCs or Tet1/Tet2 siRNA-treated PDLSCs were intraperitoneally injected into the mice 3 days after initiation of DSS treatment. DAI was scored on a scale of 0–4 by evaluating the weight loss, stool consistency/diarrhea, and presence of fecal bleeding19,43. At day 10, mice were sacrificed by CO2 euthanasia, their colons were fixed with 4% PFA, and paraffin-embedded sections were prepared for hematoxylin and eosin staining. Histological scores were blindly determined as previously described19. All animal experiments were performed under institutionally approved protocols for the use of animal research (University of Pennsylvania IACUC #805478).
Chromatin immunoprecipitation-qPCR assays
PDLSCs were cross-linked and used for each immunoprecipitation (IP). ChIP was performed using the Millipore ChIP Kit according to the manufacturer’s protocol. Branson sonifier was used to shear the chromatin. For precipitating DNA–protein complexes, Tet1 and Tet2 antibodies were used and non-specific serum immunoglobulin G was used as an isotype control. Percentage input was determined by removing an aliquot of sheared chromatin prior to IP and comparing amplification of this DNA to amplification of the precipitated chromatin. MethPrimer software was used to predict the presence of CpG islands in the DKK-1 promoter.
Hydroxymethylated DNA immunoprecipitation
IP of 5-hmC was performed using an Active Motif hMeDIP Kit. A Branson sonicator was used to sonicate DNA into short fragments (100 to 1000 base pairs (bp)), after which it was heat denatured at 95 °C for 10 min. Sonicated DNA (1 μg) was immunoprecipitated with 2.5 μg of mouse anti-5-hmC antibody (Active Motif, 1 μg/μl) and was incubated overnight at 4 °C. Then, the magnetic beads were added to the DNA–antibody mixture and isolation of immunoprecipitated DNA was performed according to the kit instructions. SYBR® Green Supermix (Bio-Rad) on a Bio-Rad CFX96 Real-Time system was used to perform qPCR, as indicated by the manufacturer’s protocol. The amount of DNA used in the IP reaction was calculated for the percentage of enrichment.
Methylation-specific PCR and OxBS-sequencing
OxBS-sequencing was used to assess DKK-1 methylation status. A highly selective chemical oxidizes 5-hmC to 5-formylcytosine (5fC) and then bisulfite treatment deformylated and deaminated 5fC to uracil, appears as thymine (T) in sequencing analysis. 5-mC is not determinate and appears as a cytosine (C) in sequencing analysis. Oxidative bisulfite conversion, which is able to produce an accurate readout distinguishing 5-mC from 5-hmC, was performed using a TrueMethyl™ Kit (Cambridge Epigenetix, UK), according to the manufacturer’s instructions44. For methylation-specific PCR, pretreated DNA was amplified with methylation-specific primers for site 1 and site 2 of DKK-1 and were sequenced as previously reported by us10.
Statistical analysis
All results are presented as the mean and standard deviation (mean ± SD) for n from 3 to 8. Independent unpaired two-tailed Student’s t tests were used for comparing between two groups. One-way analysis of variance was used for comparing between more than two groups. P values < 0.05 were considered statistically significant.
References
Ito, S. et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303 (2011).
Kim, R., Sheaffer, K. L., Choi, I., Won, K. J. & Kaestner, K. H. Epigenetic regulation of intestinal stem cells by Tet1-mediated DNA hydroxymethylation. Genes Dev. 30, 2433–2442 (2016).
Wu, X., Li, G. & Xie, R. Decoding the role of TET family dioxygenases in lineage specification. Epigenet. Chromatin 11, 58 (2018).
Szwagierczak, A., Bultmann, S., Schmidt, C. S., Spada, F. & Leonhardt, H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 38, e181 (2010).
Koh, K. P. et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213 (2011).
Tan, L. & Shi, Y. G. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 139, 1895–1902 (2012).
Yang, R. et al. Tet1 and Tet2 maintain mesenchymal stem cell homeostasis via demethylation of the P2rX7 promoter. Nat. Commun. 9, 2143 (2018).
Yang, R. et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 43, 251–263 (2015).
Whiting, D., Chung, W. O., Johnson, J. D. & Paranjpe, A. Characterization of the cellular responses of dental mesenchymal stem cells to the immune system. J. Endod. 44, 1126–1131 (2018).
Shin, C. et al. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J. Periodontal Res. 52, 135–146 (2017).
Liu, D. et al. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol. 39, 1174–1182 (2012).
Liu, Y. et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26, 1065–1073 (2008).
Zhai, Q., Dong, Z., Wang, W., Li, B. & Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 13, 152–159 (2019).
Guo, L. et al. d-Mannose enhanced immunomodulation of periodontal ligament stem cells via inhibiting IL-6 secretion. Stem Cells Int. 2018, 7168231 (2018).
Chen, C. et al. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 27, 559–577 (2017).
Shi, B. et al. Mesenchymal stem cell transplantation ameliorates Sjogren’s syndrome via suppressing IL-12 production by dendritic cells. Stem Cell Res. Ther. 9, 308 (2018).
Alex, P. et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352 (2009).
Akiyama, K. et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10, 544–555 (2012).
Kohli, R. M. & Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502, 472–479 (2013).
Xu, Y. et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol. Cell 42, 451–464 (2011).
Chen, C. et al. Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol. Med. 6, 322–334 (2014).
Liu, Y. et al. Acetylsalicylic acid treatment improves differentiation and immunomodulation of SHED. J. Dent. Res. 94, 209–218 (2015).
Lin, G. L. & Hankenson, K. D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell Biochem. 112, 3491–3501 (2011).
Luo, K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 9, a022137 (2017).
Guo, Y. et al. The Wnt inhibitor Dkk1 is required for maintaining the normal cardiac differentiation program in Xenopus laevis. Dev. Biol. 449, 1–13 (2019).
Nakatsukasa, H. et al. Loss of TET proteins in regulatory T cells promotes abnormal proliferation, Foxp3 destabilization, and IL-17 expression. Int. Immunol. 31, 335–347 (2019).
Lio, C. J. & Rao, A. TET Enzymes and 5hmC in adaptive and innate immune systems. Front. Immunol. 10, 210 (2019).
An, J. et al. Acute loss of TET function results in aggressive myeloid cancer in mice. Nat. Commun. 6, 10071 (2015).
Huang, Y. & Rao, A. Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet. 30, 464–474 (2014).
Tsagaratou, A. et al. TET proteins regulate the lineage specification and TCR-mediated expansion of iNKT cells. Nat. Immunol. 18, 45–53 (2017).
Li, Q. et al. FAM20C could be targeted by TET1 to promote odontoblastic differentiation potential of human dental pulp cells. Cell Prolif. 51, e12426 (2018).
Mahaira, L. G. et al. IGF2BP1 expression in human mesenchymal stem cells significantly affects their proliferation and is under the epigenetic control of TET1/2 demethylases. Stem Cells Dev. 23, 2501–2512 (2014).
Sajadian, S. O. et al. Vitamin C enhances epigenetic modifications induced by 5-azacytidine and cell cycle arrest in the hepatocellular carcinoma cell lines HLE and Huh7. Clin. Epigenet. 8, 46 (2016).
Squillaro, T., Peluso, G. & Galderisi, U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848 (2016).
Samsonraj, R. M. et al. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 6, 2173–2185 (2017).
Aguilera, O. et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 25, 4116–4121 (2006).
Pannone, G. et al. WNT pathway in oral cancer: epigenetic inactivation of WNT-inhibitors. Oncol. Rep. 24, 1035–1041 (2010).
Neri, F. et al. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene 34, 4168–4176 (2015).
Atlante, S. et al. Alpha-ketoglutarate dehydrogenase inhibition counteracts breast cancer-associated lung metastasis. Cell Death Dis. 9, 756 (2018).
Seo, B. M. et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155 (2004).
Zhang, Q. et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 183, 7787–7798 (2009).
Booth, M. J. et al. Oxidative bisulfite sequencing of 5-methylcytosine and 5-hydroxymethylcytosine. Nat. Protoc. 8, 1841–1851 (2013).
Acknowledgements
This work was supported by Beijing Natural Science Foundation No. 7182182 to R.Y.; Peking University Medicine Fund of Fostering Young Scholars’ Scientific and Technological Innovation (BMU2018PYB009) to T.Y., supported by “the Fundamental Research Funds for the Central Universities”; the Young Elite Scientist Sponsorship Program by CAST (YESS), 2017QNRC001; the National Natural Science Foundation of China No. 81600865 (R.Y.), 81970940 (R.Y.), 51903003 (T.Y.), and the National Science and Technology Major Project of the Ministry of Science and Technology of China No. 2018ZX10302207.
Authors’ contributions
T.Y. and R.Y. designed the experiments and wrote the manuscript. T.Y., D.L. and T.Z. performed the experiments and collected and analyzed the data. Y.Z. and S.S. designed and revised the manuscript. All co-authors approved the final version of the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Y. Shi
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, T., Liu, D., Zhang, T. et al. Inhibition of Tet1- and Tet2-mediated DNA demethylation promotes immunomodulation of periodontal ligament stem cells. Cell Death Dis 10, 780 (2019). https://doi.org/10.1038/s41419-019-2025-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-2025-z
This article is cited by
-
Aberrant DNA Methylation Profile of Dickkopf-1 in Ankylosing Spondylitis
Biochemical Genetics (2024)
-
Mesenchymal stem cells under epigenetic control – the role of epigenetic machinery in fate decision and functional properties
Cell Death & Disease (2023)
-
Membrane computing with harmony search algorithm for gene selection from expression and methylation data
Journal of Membrane Computing (2022)
-
DNA Methylation and Histone Modification in Dental-derived Mesenchymal Stem Cells
Stem Cell Reviews and Reports (2022)
-
A novel mutation of SATB2 inhibits odontogenesis of human dental pulp stem cells through Wnt/β-catenin signaling pathway
Stem Cell Research & Therapy (2021)