Abstract
Background
Reducing nivolumab dose intensity could increase patients’ life quality and decrease the financial burden while maintaining efficacy. The aims of this study were to develop a population PK model of nivolumab based on data from unselected metastatic cancer patients and to simulate extended-interval regimens allowing to maintain minimal effective plasma concentrations (MEPC).
Methods
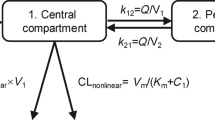
Concentration-time data (992 plasma nivolumab concentrations, 364 patients) were modeled using a two-compartment model with linear elimination clearance in Monolix software. Extended-interval regimens allowing to maintain steady-state trough concentrations (Cmin,ss) above the MEPC of 2.5 mg/L or 1.5 mg/L in >90% of patients were simulated.
Results
Increasing 3-times the dosing interval from 240 mg every two weeks (Q2W) to Q6W and 2-times from 480 mg Q4W to Q8W resulted in Cmin,ss above 2.5 mg/L in 95.8% and 95.4% of patients, respectively. 240 mg Q8W and 480 mg Q10W resulted in Cmin,ss above 1.5 mg/L in 91.0% and 91.8% of patients, respectively. Selection of a 240 mg Q6W regimen would decrease by 3-fold the annual treatment costs compared to standard regimen of 240 mg Q2W (from 78,744€ to 26,248€ in France).
Conclusions
Clinical trials are warranted to confirm the non-inferiority of extended-interval compared to standard regimen.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The Monolix code of the final popPK model and the dataset can be accessed on request.
Code availability
The Monolix code of the final popPK model and the dataset can be accessed on request.
References
Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–67.
Desnoyer A, Broutin S, Delahousse J, Maritaz C, Blondel L, Mir O, et al. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 2, immune checkpoint inhibitor antibodies. Eur J Cancer. 2020;128:119–28.
Yu PP, Eton O, Garrison LP. Challenges in assessing the clinical utility and economic value of immune checkpoint inhibitor therapies of Cancer. J Immunother Cancer. 2019;7:235.
Wesevich A, Goldstein DA, Paydary K, Peer CJ, Figg WD, Ratain MJ. Interventional pharmacoeconomics for immune checkpoint inhibitors through alternative dosing strategies. Br J Cancer. 2023;1–8.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–54.
Agrawal S, Feng Y, Roy A, Kollia G, Lestini B. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer. 2016;4:72.
Chatelut E, Hendrikx JJMA, Martin J, Ciccolini J, Moes DJAR. Unraveling the complexity of therapeutic drug monitoring for monoclonal antibody therapies to individualize dose in oncology. Pharmacol Res Perspect. 2021;9:e00757.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30.
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–7.
FDA. Clinical pharmacology and biopharmaceutic review (nivolumab). Application Number 125554Orig1s000. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125554Orig1s000ClinPharmR.pdf.
Ratain MJ, Goldstein DA. Time is Money: Optimizing the Scheduling of Nivolumab. Am Soc Clin Oncol; 2018;36:3074–6.
Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E, et al. Clinical Pharmacology and Interplay of Immune Checkpoint Agents: A Yin-Yang Balance. Ann Rev. 2021;61:85–112.
Kimura H, Sone T, Murata A, Koba H, Tambo Y, Hara J, et al. Long-lasting shrinkage in tumor mass after discontinuation of nivolumab treatment. Lung Cancer. 2017;108:7–8.
Kimura H, Araya T, Yoneda T, Shirasaki H, Kurokawa K, Sakai T, et al. Long-lasting responses after discontinuation of nivolumab treatment for reasons other than tumor progression in patients with previously treated, advanced non-small cell lung cancer. Cancer Commun. 2019;39:1–5.
Mari R, Guerin M, Vicier C, Walz J, Bonnet N, Pignot G, et al. Durable disease control and refractory bullous pemphigoid after immune checkpoint inhibitor discontinuation in metastatic renal cell carcinoma: A case report. Front Immunol. 2022;13:984132.
Ricciuti B, Metro G, Baglivo S, Colabrese D, Chiari R, Bennati C, et al. Long-Lasting Response to Nivolumab and Immune-Related Adverse Events in a Nonsquamous Metastatic Non–Small Cell Lung Cancer Patient. J Thorac Oncol. 2017;12:e51–5.
Yatsuda Y, Hirose S, Ito Y, Onoda T, Sugiyama Y, Nagafuchi M, et al. A Durable Response after the Discontinuation of Nivolumab in an Advanced Gastric Cancer Patient. Intern Med. 2021;60:1011–7.
Hirsch I, Goldstein DA, Tannock IF, Butler MO, Gilbert DC. Optimizing the dose and schedule of immune checkpoint inhibitors in cancer to allow global access. Nat Med. 2022;28:2236–7.
Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients. Cancer Res Treat. 2020;53:339–54.
Gravis G, Marino P, Olive D, Penault-LLorca F, Delord JP, Simon C, et al. A non-inferiority randomized phase III trial of standard immunotherapy by checkpoint inhibitors vs. reduced dose intensity in responding patients with metastatic cancer: the MOIO protocol study. BMC Cancer. 2023;23:1–10.
Marin C, Khoudour N, Millet A, Lebert D, Bros P, Thomas F, et al. Cross-validation of a multiplex lc-ms/ms method for assaying mabs plasma levels in patients with cancer: A gpco-unicancer study. Pharmaceuticals. 2021;14:796.
Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6:58–66.
Boudou-Rouquette P, Arrondeau J, Gervais C, Durand JP, Fabre E, De Percin S, et al. Development and validation of a host-dependent, PDL1-independent, biomarker to predict 6-month progression-free survival in metastatic non-small cell lung cancer (mNSCLC) patients treated with anti-PD1 immune checkpoint inhibitors (ICI) in the CERTIM Coho. EBioMedicine. 2021;73:103630.
Peer CJ, Heiss BL, Goldstein DA, Goodell JC, Figg WD, Ratain MJ. Pharmacokinetic Simulation Analysis of Less Frequent Nivolumab and Pembrolizumab Dosing: Pharmacoeconomic Rationale for Dose Deescalation. J Clin Pharmacol. 2022;62:532–40.
Cloesmeijer ME, van den Oever HLA, Mathôt RAA, Zeeman M, Kruisdijk-Gerritsen A, Bles CMA, et al. Optimising the dose of clonidine to achieve sedation in intensive care unit patients with population pharmacokinetics. Br J Clin Pharmacol. 2020;86:1620–31.
Economique A HAUTE AUTORITÉ DE SANTÉ EVALUER LES TECHNOLOGIES DE SANTÉ Opdivo (nivolumab) Cancer urothélial infiltrant le muscle à haut risque de récidive.
Zhang J, Sanghavi K, Shen J, Zhao X, Feng Y, Statkevich P, et al. Population Pharmacokinetics of Nivolumab in Combination With Ipilimumab in Patients With Advanced Malignancies. CPT Pharmacometrics Syst Pharmacol. 2019;8:962–70.
Osawa M, Hasegawa M, Bello A, Roy A, Hruska MW. Population pharmacokinetics analysis of nivolumab in Asian and non-Asian patients with gastric and gastro-esophageal junction cancers. Cancer Chemother Pharmacol. 2019;83:705–15.
Hurkmans DP, Basak EA, Van Dijk T, Mercieca D, Schreurs MWJ, Wijkhuijs AJM, et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J Immunother Cancer. 2019;7:192.
Hamuro L, Statkevich P, Bello A, Roy A, Bajaj G. Nivolumab Clearance is Stationary in Patients with Resected Melanoma on Adjuvant Therapy: Implications of Disease Status on Time-Varying Clearance. Clin Pharmacol Ther. 2019;106:1018–27.
Zhang J, Cai J, Bello A, Roy A, Sheng J. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Chinese Patients With Previously Treated Advanced Solid Tumors, Including Non–Small Cell Lung Cancer. J Clin Pharmacol. 2019;59:1415–24.
Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of Time-Varying Clearance of Nivolumab With Disease Dynamics and Its Implications on Exposure Response Analysis. Clin Pharmacol Ther. 2017;101:657–66.
Yoo SH, Keam B, Kim M, Kim SH, Kim YJ, Kim TM, et al. Low-dose nivolumab can be effective in non-small cell lung cancer: Alternative option for financial toxicity. ESMO Open. 2018;3:e000332.
Patil VM, Noronha V, Menon N, Rai R, Bhattacharjee A, Singh A, et al. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. J Clin Oncol. 2023;41:222–32.
Merrick S, Nankivell M, Quartagno M, Clarke CS, Joharatnam-Hogan N, Waddell T, et al. REFINE (REduced Frequency ImmuNE checkpoint inhibition in cancers): A multi-arm phase II basket trial testing reduced intensity immunotherapy across different cancers. Contemp Clin Trials. 2023;124:107030.
Tannock IF, Ratain MJ, Goldstein DA, Lichter AS, Rosner GL, Saltz LB. Near-Equivalence: Generating Evidence to Support Alternative Cost-Effective Treatments. J Clin Oncol. 2021;39:950–5.
Marolleau S, Mogenet A, Boeri C, Hamimed M, Ciccolini J, Greillier L. Killing a fly with a sledgehammer: Atezolizumab exposure in real-world lung cancer patients. CPT Pharmacometrics Syst Pharmacol. 2023;12:1795–803.
Peer CJ, Schmidt KT, Arisa O, Richardson WJ, Paydary K, Goldstein DA, et al. In Silico Re-Optimization of Atezolizumab Dosing Using Population Pharmacokinetic Simulation and Exposure–Response Simulation. J Clin Pharmacol. 2023;63:672–80.
Ratain MJ, Peer CJ, Figg WD, Goldstein DA. Dose Optimization of Pembrolizumab: Less May Be More. Clin Pharmacol Ther. 2022;111:993–993.
Millet A, Khoudour N, Bros P, Lebert D, Picard G, Machon C, et al. Quantification of nivolumab in human plasma by LC-MS/HRMS and LC-MS/MS, comparison with ELISA. Talanta. 2021;224:121889.
Author information
Authors and Affiliations
Contributions
Wrote Manuscript: AP, BP, BB. Designed Research: AP, JAl, FG, BB. Performed Research: AP, GB, BP, DB, JAr, PB-R, M-CB, J-ES, XD, MV, NK, SG, SA, OH, JAl, MW, FG, BB. Analyzed Data: AP, GB, BP, BB.
Corresponding author
Ethics declarations
Competing interests
AP received speaking fees from Bristol Myers Squibb, Eisai and Pierre Fabre Oncology. BP is employed by PhinC Development (Contract Research Organization). J-ES participated in advisory boards, consultancy or received grants from Bristol Myers Squibb, Novartis, AstraZeneca, CRC-Oncology, Servier, IPSEN, Eisai and BeiGene. SG participated in advisory board, consultancy or received grants from Janssen, Bristol Myers Squibb, Pierre Fabre and Sanofi. OH received honoraria from Sanofi, Bayer, MSD, Bristol Myers Squibb, Ipsen, Pfizer, Eisai, Janssen, AstraZeneca and Merck. JAl participated to advisory boards, consultancy or received grants from Astra Zeneca, GSK, MSD, Eisai, Novartis, Janssen. PB-R received travel fees from Pharmamar, Pfizer and grants from Ipsen. MW participated to advisory boards, consultancy or received grants from MSD, Bristol Myers Squibb, Novartis, AstraZeneca, Sanofi, Janssen and Pfizer. JAr participed in advisory board or speaking and received travel fees from Bristol Myers Squibb, Takeda, Pfizer and AstraZeneca. BB received consulting and speaking fees from Bristol Myers Squibb, Clovis Oncology, Janssen, Pierre Fabre, Pfizer and Promise. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board for retrospective analysis of the data collected as part of routine clinical practice and was conducted according to the declaration of Helsinki. Patients provided written informed consent (Cochin University Hospital: CERTIM cohort, Pitié-Salpétrière Hospital: MASC cohort, NCT04637672).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puszkiel, A., Bianconi, G., Pasquiers, B. et al. Extending the dosing intervals of nivolumab: model-based simulations in unselected cancer patients. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02659-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02659-x