Abstract
Background
Knowledge about thrombocytopenia among patients with solid tumors is scarce. We examined the risk of thrombocytopenia among patients with solid tumors and its association with adverse outcomes.
Methods
Using Danish health registries, we identified all patients with incident solid tumors from 2015-2018 (n = 52,380) and a platelet count measurement within 2 weeks prior to or on their cancer diagnosis date. The risk of thrombocytopenia was categorized as grades 0 (any platelet count × 109/L): <150; 1: <100; 2: <75; 3: <50; 4: <25, and 5: <10. To study the outcomes, each patient with thrombocytopenia was matched with up to five cancer patients without thrombocytopenia by age, sex, cancer type, and stage. Cox regression was used to compute hazard ratios (HRs) of bleeding, transfusion, or death, adjusting for confounding factors.
Results
The 1-year risk of thrombocytopenia was 23%, increasing to 30% at 4 years. This risk was higher in patients receiving chemotherapy (43% at 1 year and 49% at 4 years). Overall, patients with thrombocytopenia had higher 30-days rates of bleeding (HR = 1.72 [95% confidence interval, CI: 1.41–2.11]). Thrombocytopenia was also associated with an increased rate of transfusion, and death, but some of the risk estimates were imprecise.
Conclusions
The risk of thrombocytopenia was substantial among patients with solid tumors and associated with adverse outcomes.
Similar content being viewed by others
Introduction
Thrombocytopenia occurs frequently among patients with cancer [1]. Contributing factors are multifactorial and include use of various drugs, bleeding, tumor involvement of the bone marrow, microangiopathic disorders, disseminated intravascular coagulation, and immune disorders [1, 2].
Despite the increasing number of cancer survivors [3], knowledge is scarce about risks and prognosis related to thrombocytopenia in the routine clinical care setting. The incidence of thrombocytopenia in a population of solid tumor patients treated with chemotherapy was 10–13% in two studies based on large United States electronic medical record and claims datasets [4, 5]. In another study of adult solid tumor patients receiving chemotherapy, the 3-month risk of thrombocytopenia was 13% [6]. Thrombocytopenia can have important clinical consequences, such as postponement of or reduction in cancer treatment dose. Moreover, some prior studies found that thrombocytopenia was associated with increased risk of bleeding [7]. Prior studies’ limitations include inability to track patients across transitions between sites of care and insurance coverage. Further, the studies used broad and inconsistent cutoffs for thrombocytopenia, making it infeasible to disentangle the associations between different severities of thrombocytopenia and bleeding risk [7]. Studies were also small in size, were published more than 10 years ago, or were based on diagnosis codes, which do not reflect the biological trajectories of platelet count measures [7]. Finally, prior evidence focused on thrombocytopenia among hematologic cancer patients [7], and it is questionable whether this knowledge also applies to solid tumor patients. Firmer evidence is therefore needed about the burden of thrombocytopenia among solid tumor patients within a uniform population-based setting.
This would help to guide follow-up programs and to understand the potential impact of platelet transfusion and emerging drug therapies for thrombocytopenia. Further, it provides the foundation for guidelines on prevention and treatment strategies for solid tumor patients. We examined the occurrence, risk factors, and adverse clinical outcomes of thrombocytopenia among solid tumor patients with incident cancer and among solid tumor patients starting chemotherapy.
Methods
Study design and settings
This nationwide population-based cohort study was conducted in Denmark, which has a government-funded healthcare system providing free access to healthcare for all residents [8, 9]. Central personal registry numbers were used to link data from the Danish Cancer Registry [10], the Danish Register of Laboratory Results for Research [11], the Danish National Patient Registry [12], and the Danish Civil Registration System [13]. These data sources are described in Supplementary Table 1. According to Danish legislation, approval from an ethics committee is not required for registry-based studies. The study was reported to the Danish Data Protection Agency (record no.: 1819).
Study cohorts
The first study cohort consisted of all patients diagnosed with incident solid tumors between 2015 and 2018 who were ≥18 years and had a platelet count measurement within the 2 weeks prior to or on their cancer diagnosis date. The second cohort comprised patients who started chemotherapy at any time following their incident cancer diagnosis.
Thrombocytopenia, anemia, neutropenia, and leukopenia
Clinical data were linked with routine laboratory test results in the primary care and hospital settings. Outcomes were defined according to the Common Terminology Criterial for Adverse Events by National Cancer Institute adapted for real-world data. Based on platelet count levels, thrombocytopenia was categorized as present/absent and by severity as grades 0 (any platelet count × 109/L): <150; 1: <100 × 109/L; 2: <75 × 109/L; 3: <50 × 109/L; 4: <25 × 109/L, and 5: <10 × 109/L. Isolated thrombocytopenia was considered, i.e., absence of anemia, neutropenia, or leukopenia one day prior to or on the date of platelet count measurement. Combinations of “penias” were defined as thrombocytopenia with anemia only, thrombocytopenia with anemia and neutropenia, and thrombocytopenia with neutropenia only. Each combination of conditions had to be present on the same date ±1 day.
To contextualize risk estimates for thrombocytopenia, we also examined the risk of:
-
Anemia: Any and by severity (grade 1: Hemoglobin <lower normal limit; grade 2: <6.2-mmol/L; and grade 3: <4.9 mmol/L.
-
Neutropenia: Any and by severity (grade 1: <lower normal limit; grade 2: <1.5 × 109/L; grade 3: <1.0 × 109/L; grade 4: <0.5 × 109/L; grade 5: <0.5 × 109/L and death within 1 week after observation.
-
Leukopenia: Leukocytes <4 × 109/L.
Prognosis associated with thrombocytopenia
Each solid tumor patient with thrombocytopenia was matched with up to 5 solid tumor patients without thrombocytopenia according to age, sex, cancer type, cancer stage, and duration of cancer/duration of chemotherapy. Outcomes assessed during 30 days of follow-up were any bleeding leading to hospitalization; any transfusion, including platelet transfusion, red blood cell transfusion, or plasma transfusion; and all-cause mortality. Site-specific bleeding leading to hospitalization was also examined. This included spontaneous and traumatic intracranial bleeding, respiratory tract, gastrointestinal, urinary tract, hemorrhagic cystitis, or anemia from acute bleeding.
Covariates
We obtained data on age group, sex, year of study entry, cancer stage, bone metastasis, liver metastasis, solid cancer type, and Charlson Comorbidity Index (CCI) score. Medical conditions were chronic liver disease, disseminated intravascular coagulation, immune thrombocytopenia, and hemolytic uremic syndrome/thrombotic uremic syndrome. All available primary and secondary hospital-based discharge diagnoses (except emergency room diagnoses) prior to the index date, but after 1977, were used.
Statistical analyses
The index date was the date of incident cancer diagnosis or the date of chemotherapy initiation. All patients contributed risk time from their index date until emigration, occurrence of an outcome of interest, death, or end of the study period (31 December 2018), whichever came first. Patients could be included in both the cohort of all incident cancer patients and the cohort of patients who commenced chemotherapy. We tabulated patient characteristics for the covariates described previously. The prevalence of thrombocytopenia, anemia, neutropenia, and leukopenia was calculated using the most recent observation within 2 weeks prior to or on the index date. All analyses were performed separately for the cohort of incident solid tumor patients and for the cohort of solid tumor patients starting chemotherapy. All definitions used in the study are provided in Supplementary Tables 2–5. We tabulated the proportion of cancer patients with a platelet count measurement using different time windows to evaluate whether the patients were captured by the laboratory database. This was done by study period, Danish region, age group, and cancer type. To ensure compliance with the data protection regulations, we rounded all the presented patient numbers to the nearest five.
Risk of thrombocytopenia
We graphically illustrated the cumulative risk of thrombocytopenia, accounting for the competing risk of death [14]. We also calculated 90-day, 1-year, and 4-year risk estimates. In the overall analysis, only patients with normal levels of the individual biomarkers on their index date were considered “at risk”. In analyses of severity according to grade, patients with low-grade levels at baseline could be “at risk” of developing more severe grades during follow-up. Thus, patients were “at risk” for different outcome categories and for a more severe grade within each outcome category. For example, a patient may have experienced a higher grade of thrombocytopenia before a lower grade was observed (e.g., grade 4 before grade 2). In the grade 2 analysis, we terminated follow-up on the date of the grade 4 observation, assuming that they experienced grade 2 thrombocytopenia just before grade 4.
Further, to evaluate the burden of thrombocytopenia among affected patients, we tabulated the median numbers of “episodes” during follow-up. As well, median duration was calculated as the number of days between the initial observation and occurrence of a measurement within the normal range, or death, emigration, or end of follow-up, whichever came first. In this analysis, patients could contribute more than one episode.
Risk factors for thrombocytopenia
We assessed risk factors for thrombocytopenia according to baseline characteristics. Risk factors included age (reference group: 18–30 years), sex (reference group: male), cancer stage (reference group: localized), cancer type (reference group: breast cancer), CCI score (reference group: CCI score of 0), and cancer treatment within 30 days before and 7 days after the index date (reference group: non-receipt of a specific treatment). For this analysis, the index date was 7 days after the cancer diagnosis/chemotherapy date, to avoid immortal time bias. For solid tumor patients initiating chemotherapy, additional risk factors included type of chemotherapy (focusing on the 10 most commonly used drug regimens). 90-day, 1-year, and 4-year hazard ratios (HRs) with 95% confidence intervals (CIs) were computed using a multivariable Cox proportional hazards regression model. The HRs were both unadjusted and adjusted by age, sex, cancer stage, cancer type, CCI score, and cancer treatment.
Adverse outcomes following thrombocytopenia
To address the prognostic impact of thrombocytopenia on adverse outcomes, we sampled five comparators without thrombocytopenia (with replacement) for each patient who experienced thrombocytopenia [15]. For the prognosis part of the study, patients with thrombocytopenia (platelet count level ×109/L) were categorized into mutually exclusive groups: grade 0 = 100–149, grade 1 = 75–99, grade 2 = 50–74, grade 3 = 25–49, grade 4 = < 25-10, and grade 5 = < 10 and were followed for a maximum of 30 days. These analyses included only patients having at least 30 days between their index date and administrative study end (31 December, 2018). Incidence rates of study outcomes per 1000 person–years were calculated. Cox proportional hazards regression models were fitted to estimate HRs, adjusting for CCI score and matching factors, by design. In additional analyses, we examined associations between different combinations of thrombocytopenia, anemia, and neutropenia and all-cause mortality.
Results
We identified 115,460 adult patients with incident solid tumors (Fig. 1). After restricting this population to patients with a platelet count measurement within 2 weeks prior to or on the index date, the cohort comprised 52,380 patients (median age = 70 years, interquartile range: 61-77 years). Changing the time windows for a platelet count measurement showed that around 95% of the patients had a measurement at any time points (Supplementary Table 6). Among patients with solid tumors, 32% had localized disease, 17% had regional spread, 27% had metastasis, and 25% had an unknown or missing cancer stage. The most common cancer types were gastrointestinal cancers (32%), respiratory cancers (23%), urogenital cancers (20%), and breast cancer (13%). The majority (56%) of patients had no comorbidity (evaluated by their CCI score) at study inclusion. Among 4%, thrombocytopenia of any grade was observed during the 2 weeks preceding study inclusion (grade 0: 3%, grade 1: 1%, and grades 2–4: 0%) (Table 1). On the cancer diagnosis date, anemia was present among 36% of solid tumor patients.
For the cancer cohort, the proportion of patients with a platelet count measurement was 65%. This number varied by geographical region. Capital Region: 74%, Central Region: 71%, Northern Region: 67%, Zealand Region: 67%, Southern Region: 60%. For the cancer cohort, the distribution of cancer patients with a platelet count measurement was stable during the study period in the Capital Region, Central Region, Northern Region, and Zealand Region. In Southern Region, the proportion of cancer patients with a platelet count measurement was 22% in 2015, while it was 71% in 2016, 72% in 2017, and 72% in 2018. For the chemotherapy cohort, the proportion of patients with a platelet count measurement was 96%.
Risk of thrombocytopenia
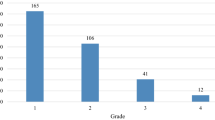
The 90-day, 1-year, and 4-year risks of new-onset thrombocytopenia were 14% (95% CI: 14–15%), 23% (95% CI: 23–24%), and 30% (95% CI: 29–30%), respectively (Fig. 2 and Supplementary Table 7). Among those who initiated chemotherapy (n = 32,680), the 90-day, 1-year, and 4-year risks of thrombocytopenia were 34% (95% CI: 33–34%), 43% (95% CI: 42–43%), and 49% (95% CI: 48–49%), respectively. (Fig. 2 and Supplementary Table 7). Grades 0–2 thrombocytopenia occurred more frequently than grades 3-4. The number and duration of episodes of thrombocytopenia are shown in Supplementary Table 8 (median episodes = 1, interquartile range: 1–3 episodes and median duration = 16 days, interquartile range: 8–41 days).
Risk of anemia, neutropenia, and leukopenia
Among solid tumor patients with incident cancer, the 90-day, 1-year, and 4-year risks of new-onset anemia were 52% (95% CI: 51–52%), 64% (95% CI: 64–65%), and 72% (95% CI: 72–73%), respectively (Fig. 1 and Supplementary Table 8). Neutropenia and leukopenia occurred less frequently. The number and duration of episodes of anemia, neutropenia, and leukopenia are shown in Supplementary Table 8 and the risk estimates in cancer patients initiating chemotherapy are presented in Supplementary Fig. 1.
Risk factors for thrombocytopenia
Rates of thrombocytopenia were increased in patients with a high burden of comorbidity, in men, and in patients with an advanced cancer (Supplementary Tables 9 and 10). The risk of thrombocytopenia was also more pronounced in some types of cancer (e.g., bone, gastrointestinal, and respiratory cancers compared with breast cancer), and in the 90 days following radiation therapy. Advanced age and immunotherapy were not associated with increased risk of thrombocytopenia. The effect estimates of individual chemotherapy regimens for the risk of thrombocytopenia were generally imprecise, but some heterogeneity was observed.
Adverse outcomes following thrombocytopenia
Solid tumor patients with thrombocytopenia had higher rates of any bleeding leading to hospitalization than patients without thrombocytopenia (overall adjusted HR = 1.67 [95% CI: 1.36–2.04], grade 0: adjusted HR = 1.54 [95% CI: 1.22–1.95], grade 1: adjusted HR = 2.03 [95% CI: 1.18–3.49], and grade 2: adjusted HR = 1.58 [95% CI: 0.72–3.45]). Grades 3-4 thrombocytopenia also showed an increased rate of hospitalization for bleeding, but estimates were imprecise (Table 2). For grade 5, we detected too few outcomes to estimate the hazard ratios. The overall estimate was similar in the population of solid tumor patients starting chemotherapy (adjusted HR = 1.48 [95% CI: 1.09–2.00]). Site-specific bleeding results were generally imprecise (Supplementary Tables 11 and 12).
All grades of thrombocytopenia were associated with an increased rate of transfusion (overall for incident solid tumors: adjusted HR = 6.96 [95% CI: 4.41–10.99]) and with death (adjusted HR = 3.54 [95% CI: 3.24–3.87]) (Tables 2 and 3). The association with death generally increased in magnitude with decreasing platelet count levels. The adjusted HRs of death according to combinations of thrombocytopenia, anemia, and neutropenia are shown in Supplementary Table 13. The HRs were higher for thrombocytopenia in combination with other penias than for isolated thrombocytopenia. However, even patients with low-grade isolated thrombocytopenia had a higher mortality rate than patients without thrombocytopenia. Using 1:1 matching led to more imprecise estimates, but the results were broadly unchanged (Supplementary Tables 14-15).
Discussion
Our study provided insights into thrombocytopenia occurrence, risk factors, and prognosis among incident solid tumor patients in a population-based setting. The risk of thrombocytopenia was substantial, particularly among patients starting chemotherapy. Risk factors included male sex, advanced stage of cancer, and a high burden of comorbidity. Advanced cancer has been reported to be a risk factor for thrombocytopenia in cancer patients [1]. Old age and many comorbidities such as autoimmune diseases and liver diseases are risk factors for thrombocytopenia [16, 17]. All grades of thrombocytopenia were associated with an increased rate of bleeding leading to hospitalization, transfusion, and death.
Our overall findings are not directly comparable to prior studies, as different definitions of thrombocytopenia were used. As well, earlier research primarily focused on selected patient populations and on chemotherapy-induced thrombocytopenia. For example, one study based on data from the United States Truven Health Analytics MarketScan Database and the IQVIA Real-World Data PharMetrics Database identified all adult patients with primary solid tumors or non-Hodgkin lymphoma who received a course of chemotherapy during 2011-2015 [4]. The incidence of a diagnosis code for thrombocytopenia during the chemotherapy course was 9.7% among all patients in the study population, ranging from 6.1% among patients receiving a cyclophosphamide-based regimen to 13.5% among those receiving a gemcitabine-based regimen [4]. In another study conducted in the United States that examined adult patients with solid tumors treated in outpatient oncology clinics during 2000-2007, the prevalence of thrombocytopenia during the course of chemotherapy ranged from 21.9% in patients treated with taxane-based regimens to 64.2% in patients treated with gemcitabine-based regimens [5]. Our findings on the risk of thrombocytopenia significantly expand the literature, suggesting that the routine clinical care burden of thrombocytopenia among patients with solid tumors and in those receiving chemotherapy is somewhat higher than anticipated.
It is well known that cancer patients are at increased risk of bleeding [18, 19]. The mechanisms are multifactorial, involving anticoagulant therapy, hyperfibrinolysis, tumor invasion, insufficient production of coagulation factors, and presence of other risk factors for bleeding. Although platelet count levels play a pivotal role in bleeding among cancer patients, there is a paucity of data on their prognostic impact. Several studies examined the association between platelet count level and bleeding among patients with hematological cancers [20,21,22,23,24,25] or in mixed populations encompassing both hematologic malignancies and solid tumors [26,27,28,29], but evidence is limited among patients with solid tumors [30,31,32,33,34]. Some research on patients with non-hematological cancers, mostly experimental or in vitro studies, focused on estimating the lowest platelet count resulting in effective hemostasis [35,36,37,38]. For example, one study demonstrated that thrombin generation [38] was maintained as long as the platelet count was above 10 × 109/L. At lower levels, thrombin generation declined proportionally to the platelet count. Unfortunately, our data did not allow us to identify the platelet count threshold for effective hemostasis.
Our study size and population coverage, as well as the longitudinal nature and completeness of follow-up characterizing the Danish data sources, are strengths of our study. In addition, the validity of the registry diagnoses that we used in our analyses is high [12]. Laboratory measurements also were performed in accredited hospital laboratories and are thus accurate [11].
Study limitations must be noted. Our data indicated that some clinicians did not measure the platelet count level within the 2 weeks before the record of a cancer diagnosis in the Cancer Registry or, in a few cases, it could indicate that the diagnosis date record was incorrect. Reassuringly, when changing the time window of measurements and when restricting to those who received chemotherapy, our data suggested that the laboratory database is virtually complete. Further, ex vivo agglutination of platelets, while rare, is reported as a text-written result and therefore was not captured by our data sources [11]. A limitation was that a large proportion of patients had missing data on cancer stage. Finally, in the prognosis part of the study, matching was done with replacement, and a substantial number of comparison cohort members were selected several times, leading to CIs that appeared too narrow. Moreover, we did not have data on the severity and specific type of surgical procedures, over the counter medication use, vaccinations, and life style factors. For example, males have been reported to have a lower age-adjusted mean platelet count than females. The reduced platelet count has been suggested to reflect sex difference in hemostasis and effect of smoking on the hemostatic system [39]. Therefore, residual confounding cannot entirely be ruled out.
Our findings may have clinical implications. As there are no drugs for treating thrombocytopenia among patients with cancer, current treatment options are to eliminate/reduce the underlying cause of thrombocytopenia or to provide platelet transfusions. It must be kept in mind that our data were observational and reflected what occurred in routine clinical practice. It is likely that patients with severe thrombocytopenia already received platelet transfusions as prophylactic treatment, which may have blurred and underestimated the true associations between platelet count levels and bleeding. Nonetheless, our data implied that thrombocytopenia is a serious clinical condition in cancer patients.
Data availability
Data presented in this study were obtained from Danish registries. Due to data protection rules, we are not allowed to share individual-level data. Other researchers who fulfill the requirements set by the data providers could obtain similar data.
References
Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133:S63–69.
Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803–18.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
Weycker D, Hatfield M, Grossman A, Hanau A, Lonshteyn A, Sharma A, et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer. 2019;19:151.
Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000-2007. Clin Ther. 2009;31:2416–32.
Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106:662–72.
Larsen JB, Hojbjerg JA, Hvas AM. The role of platelets in cancer-related bleeding risk: a systematic review. Semin Thromb Hemost. 2020;46:328–41.
Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–91.
Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–54.
Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry-history, content, quality and use. Dan Med Bull. 1997;44:535–9.
Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469–75.
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90.
Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9.
Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–70.
Heide-Jørgensen U, Adelborg K, Kahlert J, Sørensen HT, Pedersen L. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol. 2018;10:1325–37.
Stasi R. How to approach thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2012;1:191–7.
Lambert MP. Platelets in liver and renal disease. Hematology Am Soc Hematol Educ Program. 2016;1:251–5.
Inagaki J, Rodriguez V, Bodey GP. Proceedings: causes of death in cancer patients. Cancer. 1974;33:568–73.
Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4.
AlRstum ZA, Huynh TT, Huang SY, Pisimisis GT. Risk of bleeding after ultrasound-guided jugular central venous catheter insertion in severely thrombocytopenic oncologic patients. Am J Surg. 2019;217:133–7.
Estcourt LJ, Stanworth SJ, Harrison P, Powter G, McClure M, Murphy MF, et al. Prospective observational cohort study of the association between thromboelastometry, coagulation and platelet parameters and bleeding in patients with haematological malignancies- the ATHENA study. Br J Haematol. 2014;166:581–91.
Leinoe EB, Hoffmann MH, Kjaersgaard E, Nielsen JD, Bergmann OJ, Klausen TW, et al. Prediction of haemorrhage in the early stage of acute myeloid leukaemia by flow cytometric analysis of platelet function. Br J Haematol. 2005;128:526–32.
Russell L, Holst LB, Kjeldsen L, Stensballe J, Perner A. Risks of bleeding and thrombosis in intensive care unit patients with haematological malignancies. Ann Intensive Care. 2017;7:119.
Stanworth SJ, Dyer C, Casbard A, Murphy MF. Feasibility and usefulness of self-assessment of bleeding in patients with haematological malignancies, and the association between platelet count and bleeding. Vox Sang. 2006;91:63–69.
Uhl L, Assmann SF, Hamza TH, Harrison RW, Gernsheimer T, Slichter SJ. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017;130:1247–58.
Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–46.
Elting LS, Martin CG, Kurtin DJ, Cantor SB, Rubenstein EB, Rodriguez S, et al. The Bleeding Risk Index: a clinical prediction rule to guide the prophylactic use of platelet transfusions in patients with lymphoma or solid tumors. Cancer. 2002;94:3252–62.
Patell R, Gutierrez A, Rybicki L, Khorana AA. Identifying predictors for bleeding in hospitalized cancer patients: a cohort study. Thromb Res. 2017;158:38–43.
Ryu JA, Lee D, Yang JH, Chung CR, Park CM, Suh GY, et al. Spontaneous intracranial haemorrhage in critically ill patients with malignancies. Support Care Cancer. 2016;24:2971–8.
Huang CC, Chao PJ, Guo SS, Wang CJ, Luo HL, Su YL, et al. Developing a multivariable normal tissue complication probability model to predict late rectal bleeding following intensity-modulated radiation therapy. J Cancer. 2019;10:2588–93.
Tagawa ST, Dorff TB, Rochanda L, Ye W, Boyle S, Raghavan D, et al. Subclinical haemostatic activation and current surgeon volume predict bleeding with open radical retropubic prostatectomy. BJU Int. 2008;102:1086–91.
Tanaka S, Ishihara K, Watanabe C, Ohno K, Yamamoto T. Hepatectomy for hepatocellular carcinoma in patients with severe thrombocytopenia. Hepatogastroenterology. 2011;58:1316–20.
Wang S, Ye Q, Tu J, Song Y. The location, histologic type, and stage of lung cancer are associated with bleeding during endobronchial biopsy. Cancer Manag Res. 2018;10:1251–7.
Zhou L, Zhang LZ, Wang JY, Li YW, Hu HD, Peng XM, et al. Perioperative safety analysis of transcatheter arterial chemoembolization for hepatocellular carcinoma patients with preprocedural leukopenia or thrombocytopenia. Mol Clin Oncol. 2017;7:435–42.
Slichter SJ, Harker LA. Thrombocytopenia: mechanisms and management of defects in platelet production. Clin Haematol. 1978;7:523–39.
Slichter SJ, LeBlanc R, Jones MK. Quantitative analysis of bleeding risk in cancer patients (PTS) prophylactically transfused at platelet (PLT) counts (CTS) of, 5000, 10,000, or 20,000. Blood. 1999;94:376a.
Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105–9.
Chantarangkul V, Clerici M, Bressi C, Giesen PL, Tripodi A. Thrombin generation assessed as endogenous thrombin potential in patients with hyper-or hypo-coagulability. Haematologica. 2003;88:547–54.
Green MS, Peled I, Najenson T. Gender differences in platelet count and its association with cigarette smoking in a large cohort in Israel. J Clin Epidemiol. 1992;45:77–84.
Funding
This study was partly funded by Amgen as an institutional grant to Aarhus University. Open access funding provided by Aarhus Universitet.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. KV and HTS acquired the data. KA, EH-P, and HTS directed the analyses, which were carried out by KV. KA wrote the initial draft. All authors contributed to the discussion and interpretation of the results, which secured the intellectual content of the manuscript. All authors accepted the final version for submission.
Corresponding author
Ethics declarations
Competing interests
KV, EH-P, and HTS declare no personal conflict of interest. Department of Clinical Epidemiology is, however, involved in studies with funding from various companies as research grants to (and administered by) Aarhus University, including this study. Mary Clouser and Hossam Saad are employees of Amgen. Kasper Adelborg is now an employee of Novo Nordisk.
Ethics approval
According to Danish legislation, approval from an ethics committee is not required for registry-based studies. The study was reported to the Danish Data Protection Agency (record no. 1819).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adelborg, K., Veres, K., Horváth-Puhó, E. et al. Risk and adverse clinical outcomes of thrombocytopenia among patients with solid tumors—a Danish population-based cohort study. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02630-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02630-w