Abstract
We describe 1000 patients with essential thrombocythemia seen at the Mayo Clinic between 1967 and 2023: median age 58 years (18–90), females 63%, JAK2/CALR/MPL-mutated 62%/27%/3%, triple-negative (TN) 8%, extreme thrombocytosis (ExT; platelets ≥1000 × 109/L) 26%, leukocytosis (leukocyte count >11 × 109/L) 20%, and abnormal karyotype 6%. JAK2-mutated patients were older (median 71 years), and CALR mutated (52 years), and TN (50 years) younger (p < 0.01). Female gender clustered with TN (73%) and JAK2 (69%) vs. CALR/MPL (49%/47%) mutations (p < 0.01). ExT clustered with CALR (type-2 more than type-1) and TN and leukocytosis with JAK2 mutation (p < 0.01). In multivariable analysis, risk factors for overall survival were older age (p < 0.01), male gender (HR 1.8), absolute neutrophil count (ANC) ≥ 8 × 109/L (HR 1.6), absolute lymphocyte count (ALC) < 1.7 × 109/L (HR 1.5), hypertension (HR 1.7), and arterial thrombosis history (HR 1.7); for leukemia-free survival, ExT (HR 2.3) and abnormal karyotype (HR 3.1); for myelofibrosis-free survival, ANC ≥ 8 × 109/L (HR 2.3) and MPL mutation (HR 3.9); for arterial thrombosis-free survival, age ≥60 years (HR 1.9), male gender (HR 1.6), arterial thrombosis history (HR 1.7), hypertension (HR 1.7), and JAK2 mutation (HR 1.8); for venous thrombosis-free survival, male gender (HR 1.8) and venous thrombosis history (HR 3.0). Associations between ExT and leukemic transformation and between ANC and fibrotic progression were limited to JAK2-mutated cases. Aspirin therapy appeared to mitigate both arterial (HR 0.4) and venous (HR 0.4) thrombosis risk. HR-based risk models delineated patients with median survivals ranging from 10 years to not reached and 20-year leukemia/myelofibrosis incidences from 3%/21% to 12.8%/49%. The current study provides both novel and confirmatory observations of essential thrombocythemia.
Similar content being viewed by others
Introduction
Essential thrombocythemia (ET) is one of four JAK2 mutation-prevalent myeloproliferative neoplasms (MPNs) and is characterized by a mandatory but not specific thrombocytosis (platelet count ≥450 × 109/L) that is proven or presumed to be clonal and not associated with another myeloid neoplasm, such as chronic myeloid leukemia (CML), polycythemia vera (PV), and primary myelofibrosis (PMF) [1, 2]. The latter two share common molecular and morphologic traits with ET, including JAK2, CALR, and MPL mutations (also known as MPN driver mutations); these mutations are mutually exclusive, for the most part, and their frequencies in ET are ~60% for JAK2, 25% for CALR, and 3% for MPL; of note, these three driver mutations might not be detected in ~10–15% of patients with ET, henceforth referred to as triple-negative ET [1, 3].
Prognosis in ET is generally favorable with consistent risk of thrombohemorrhagic complications and disease progression into myelofibrosis (post-ET MF) or acute myeloid leukemia (AML), also known as “blast phase MPN” [4,5,6]. Survival in ET approximates that of the general population with median estimated to exceed 30 years in patients younger than 40 years of age [7,8,9]. The recently introduced “triple A (AAA)” survival model in ET employs age, absolute neutrophil count, and absolute lymphocyte count, in order to risk-stratify patients into high, intermediate-2, intermediate-1, and low-risk groups, with respective median survivals of 8, 13.5, 20.7, and 47 years [10]. In addition, abnormal karyotype [11] and high molecular risk (HMR-ET; SF3B1, TP53) mutations [12] independently predict inferior survival in ET. Current drug therapy has not been shown to modify the natural history of the disease, and its use is primarily directed at the prevention of thrombosis, guided by thrombosis risk models that are based on thrombosis history, age, and presence of JAK2 mutation [13, 14].
Over the last half-century, the Mayo Clinic has been a center of excellence for patient care and research in MPN, under the leadership of the late Murray N. Silverstein (1928–1998); part of this decades-long experience has been assembled into previously published large natural history studies, including a 1000-patient report on primary myelofibrosis [15]. The current report includes 1000 patients with ET, seen at the Mayo Clinic between 1967 and 2023, and selected on the basis of full annotation for driver mutations; we describe presenting clinical and laboratory characteristics, frequency and outcome of post-diagnosis events, and detailed global and driver mutation-specified analyses of overall, leukemia-free, and myelofibrosis-free survival, as well as predictors for such events.
Methods
The current study includes 1000 consecutive patients with ET, who underwent evaluation at the Mayo Clinic between December 1967 and March 2023 and in whom bone marrow biopsies and driver mutation information was available for review. All cases fulfilled the ICC 2022 diagnostic criteria [16] and were fully annotated for driver mutations, while cytogenetic information was available in a subset of patients (n = 875). Patients were retrospectively recruited after institutional review board approval was obtained. In order to minimize the inadvertent inclusion of patients with masked PV [17], JAK2 mutated cases with hemoglobin level >16 g/dL in women and 16.5 g/dL in men were excluded; similarly, cases with anemia defined by sex adjusted hemoglobin level of <11 g/dL in women and <12.5 g/dL in men without an alternative explanation were also excluded, in order to avoid inadvertent inclusion of patients with prefibrotic MF [18]. Thrombosis and survival risk was assessed by the revised IPSET-thrombosis [13] and triple A survival model [10], respectively. Conventional criteria were used for definitions of major arterial and venous thrombotic events, major hemorrhage, fibrotic or leukemic transformation [4, 6, 16]. Therapeutic interventions were dependent on physician discretion and mostly included aspirin therapy in low-risk patients and the addition of cytoreductive therapy, in high-risk patients. Patients were followed until death or last follow-up, as assessed by medical records or through direct contact with patients or their physicians, with follow-up information updated in August 2023.
Comparison between categorical variables was performed by Chi-square test and continuous variables by Wilcoxon/Kruskal–Wallis tests. Cox regression analysis was used to identify risk factors for overall (OS), leukemia-free survival (LFS), myelofibrosis-free (MFFS), and thrombosis-free (TFS). The Kaplan–Meier method was used to construct time-to-event curves, which were compared by the log-rank test. P value ≤ 0.05 was considered significant. JMP Pro 17.1.0 software package, SAS Institute, Cary, NC was utilized for all analyses.
Results
Presenting characteristics
One thousand patients with ET (median age 58 years, 63% female) were fully annotated for driver mutations which included JAK2 (62%, n = 617), CALR (27%, n = 269 [type 1/type 1-like CALR, n = 149; type 2/type-2-like CALR, n = 105, CALR type indeterminate, n = 15]), MPL (3%, n = 30), or TN (8%, n = 84). Information regarding presenting clinical and laboratory features, including treatment, was available in most patients (Table 1). Median age/gender distributions for JAK2, type 1/type 1-like CALR, type 2/type 2-like CALR, MPL-mutated, and TN cases were 71 years/69% females, 53 years/50% females, 51 years/51% females, 66 years/47% females, and 50 years/73% females, respectively (p < 0.01/< 0.01). Median values for hemoglobin, platelet, and leukocyte count were 13.9 g/dL, 777 × 109/L (extreme thrombocytosis (ExT); platelets ≥1000 × 109/L in 26%) and 8.5 × 109/L (leukocyte count >11 × 109/L in 20%), respectively. Median/range values for ANC (n = 653) were 5.69 (1.54–26.5) (ANC ≥ 8 × 109/L in 17%), and ALC (n = 650) were 1.86 (0.38–5.69) (ALC < 1.7 × 109/L in 40%). Palpable splenomegaly was documented in 12% patients, microvascular symptoms in 29% and cardiovascular risk factors in 54%. Cytogenetic studies showed an abnormal karyotype in 6% with incidence rates of 7%, 4%, 3%, and 4%, in JAK2/CALR/MPL-mutated and TN cases, respectively, (p = 0.31). Treatment information was available in most patients; aspirin was initiated at the time of diagnosis in 763/908 (81%), cytoreductive therapy in 563/915 (62%), and systemic anticoagulation in 167/859 (19%) of patients.
Table 1 lists presenting features of 1000 patients with ET and highlights salient associations of genotype with phenotype. At presentation, JAK2-mutated patients compared with CALR-mutated counterparts were significantly older (median age; 71 vs 52 years; p < 0.01), displayed female preponderance (69% vs 49%; p < 0.01), higher incidence of hypertension (46 vs 36%; p < 0.01), and smoking (21% vs 15%; p = 0.02), higher hemoglobin (median Hb; 14 vs 13.6 g/dl; p < 0.01), and leukocyte count (median leukocyte count; 8.9 vs 8 × 109/l; p < 0.01), lower platelet count (median platelet count; 705 vs 955 × 109/l; p < 0.01) and higher rates of arterial (16% vs 7%; p < 0.01) and venous thrombosis history (13% vs 4%; p < 0.01). Findings were similar when JAK2-mutated cases were compared with those harboring type 1/type 1-like or type 2/type 2-like CALR mutations (Supplementary Table 1). Overall, type 1/type 1-like and type 2/type 2-like CALR-mutated patients depicted similar phenotype with the exception of higher platelet count in the presence of type 2/type 2-like CALR mutation (median; 1044 vs 890 × 109/l; p = 0.001) (Supplementary Table 1). Compared with MPL-mutated cases, JAK2-mutated patients were older (median age; 71 vs 66 years; p = 0.02), more likely to be female (69 vs 47%; p = 0.01) and displayed higher leukocyte count (median leukocyte count; 8.9 vs 7.4 × 109/l; p = 0.001). JAK2-mutated and TN patients shared similar gender distribution (predominantly female), with notable differences in age (older age for JAK2-mutated), hemoglobin level (higher in JAK2-mutated), leukocyte count (higher in JAK2-muated) and platelet count (higher in TN). On the other hand, comparison of CALR-mutated and TN patients revealed the former to be associated with male gender, higher hemoglobin level, and lower rates of arterial and venous thrombosis and major hemorrhage history (Table 1).
Major thrombosis history at or before diagnosis was present in 222 (22%) of patients, including 137 (14%) arterial, and 102 (10%) venous events. Incidence rates of major arterial/venous thrombosis for JAK2, type 1/type 1-like CALR, type 2/type 2-like CALR, MPL-mutated and TN cases were 16%/13%, 7%/5%, 7%/2%, 13%/7%, and 18%/12%, respectively. Arterial and venous thrombosis rates were significantly lower in CALR-mutated cases when compared with JAK2, whose thrombosis risk was otherwise similar to those with MPL mutation and TN (p < 0.01 and <0.01). Furthermore, advanced age, male gender, and hypertension showed an independent association with arterial thrombosis at or prior to diagnosis (Table 2). 74 of 983 (8%) patients reported major hemorrhage history with respective incidence rates of 8%, 4%, 7%, 10%, and 13%, for JAK2, type 1/type 1-like CALR, type 2/type 2-like CALR, MPL-mutated and TN cases. Notably, CALR-mutated patients were less likely to present with major hemorrhage compared to JAK2, MPL-mutated, and TN cases (5% vs 10%; p = 0.05). On the other hand, female gender was associated with higher rates of hemorrhage (76% vs 24% in female and male patients; p = 0.02) (Table 2).
Clinical course
Survival
Among 1000 patients with ET, 282 (28%) were followed until death; the median follow-up time for all patients and living patients was 8.5 years (range, 0.01–52.7) and 7.1 years (range, 0.01–52.7), respectively. Causes of death were known in 124 patients and included blastic transformation (n = 26), infection (n = 26), thrombosis (n = 16), solid tumor (n = 15), major hemorrhage (n = 8), heart failure (n = 6), myelofibrosis (n = 5), dementia (n = 4), renal failure (n = 5), hepatic failure (n = 3), injury/accident (n = 3), graft-versus host disease (n = 2) and others (n = 8).
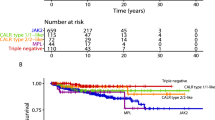
Median survival was 20.6 years with 10-year, 20-year, and 30-year survival rates of 81%, 52%, and 25%, respectively. In univariate analysis, OS appeared significantly better in type 1/type 1-like CALR, type 2/type 2-like CALR-mutated and TN patients (median 23.1/23.6/22.7 years, respectively) and worse in MPL and JAK2-mutated cases (median 16.9/17.8 years) (p = 0.03) (Fig. 1a). However, the difference in OS was no longer apparent (p = 0.39) during multivariable analysis that included age and gender, which were differentially clustered with specific driver mutations (Table 1). Moreover, univariate analysis also identified the following variables as risk factors for OS: age >70 years and 50–70 years, ANC ≥ 8 × 109/L, ALC < 1.7 g/dl, male gender, arterial thrombosis history, hypertension, abnormal karyotype, arterial thrombosis, and major hemorrhage after diagnosis. In multivariable analysis, age >70 years and 50–70 years (p < 0.01; HR 22.4/5.1, 95% CI 11.7–43.2/2.8–9.6, respectively), ANC ≥ 8 × 109/L (p < 0.01; HR 2.4, 95% CI 1.6–3.4), ALC < 1.7 g/dl (p = 0.02; HR 1.5, 95% CI 1.1–2.1), male gender (p = 0.01; HR 1.8, 95% CI 1.3–2.7), arterial thrombosis history (p = 0.01; HR 1.7, 95% CI 1.1–2.7), and hypertension (p = 0.01; HR 1.7, 95% CI 1.1–2.6) were independently predictive of inferior survival (Table 3).
a Overall survival in 985 patients with essential thrombocythemia stratified by driver mutation. b Leukemia-free survival in 985 patients with essential thrombocythemia stratified by driver mutation. c Myelofibrosis-free survival in 985 patients with essential thrombocythemia stratified by driver mutation.
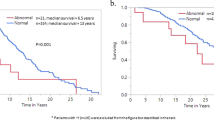
Subsequently, a three-tiered “triple A” risk stratification model was applied in 653 informative patients with HR-weighted scoring, allocating four adverse points for age >70 years, two adverse points for age 50–70 years, one adverse point each for ANC ≥ 8 × 109/L, and, ALC < 1.7 g/dl: low (0–1 point; n = 194), intermediate-1 (2–3 points; n = 277), and high/intermediate-2 (4–6 points; n = 182), with respective median survival (20-year rate) of not reached (80%), 21.3 years (60%), and 10.6 (7%) years (p < 0.0001) (Fig. 2).
Blastic transformation and fibrotic progression
At the time of last follow-up in August 2023, blastic transformations were reported in 33 patients (3%), with overall incidence, 10-year and 20-year rates of 3%, 1.5% and 7.6%, respectively; 3%/1.7%/8% for JAK2, 3%/0.9%/0.9% for type 1/type 1-like CALR, 6%/3%/6% for type 2/type 2-like CALR, 3%/0%/0.2% with MPL and 0%/0%/0% with TN. There was no significant difference in LFS among the driver mutational categories (p = 0.29) (Fig. 1b). On the other hand, LFS was significantly worse in patients with ExT; platelet count ≥1000 × 109/l (p = 0.05; HR 2.3, 95% CI 1.0–5.2) and abnormal karyotype (p = 0.03; HR 3.1, 95% CI 1.1–9.3) (Table 3). Based on the aforementioned findings, a two-tiered blastic transformation risk model was developed in 910 informative cases, allocating one adverse point each for platelet count ≥1000 × 109/l and abnormal karyotype: low (0 points; n = 604), and high (1–2 points; n = 306), with respective median LFS (20-year rate) of not reached (3%), and 36.5 years (12.8%) (p = 0.004) (Fig. 3a). It is to be noted none of the TN patients succumbed to leukemic transformation and the latter’s association with ExT was limited to JAK2-mutated cases (p = 0.0002). On the other hand, abnormal karyotype was associated with inferior LFS in CALR-mutated cases (p = 0.03).
126 patients (13%) experienced transformation to myelofibrosis with 10-year and 20-year incidence rates of 9% and 30%, respectively. Overall incidence/10-year/20-year figures for fibrotic transformation were 10%/8%/26% for JAK2-mutated, 20%/13%/36% type 1/type 1-like CALR-mutated, 15%/5%/39% type 2/type 2-like CALR-mutated, 27%/35%/63% MPL-mutated and 10%/5%/19% TN cases (p < 0.01); the difference was significant for MPL vs JAK2 (p < 0.01, HR 4.0, 95% CI 1.9–8.4), MPL vs type 1/type 1-like and type 2/type 2-like CALR (p = 0.03 and 0.01, respectively, HR 2.4, 95% CI 1.1–5.2 and HR 3.7, 95% CI 1.6–8.7), type 1/type 1-like CALR vs JAK2 (p = 0.02, HR 1.7, 95% CI 1.1–2.6), and type 1/type 1-like CALR vs TN (p = 0.03, HR 2.4, 95% CI 1.1–5.3) (Fig. 1c). Univariate analysis also identified age, male gender and ANC ≥ 8 × 109/L as risk factors for fibrotic progression (p = 0.04/0.003/0.04). Multivariable analysis that included factors that were significant in univariate analysis confirmed the independent prognostic relevance of MPL mutations (p < 0.01; HR 3.9, 95% CI 1.8–8.4), and ANC ≥ 8 × 109/L (p = 0.01; HR 2.3, 95% CI 1.2–4.3) for MFFS (Table 3). An HR-based risk model incorporating MPL mutation (2 points) and ANC ≥ 8 × 109/L (1 point) delineated patients with median MFFS ranging from not reached to 22.2 years, and 20-year myelofibrosis incidence from 12% to 49%, in low (0 points) and high-risk (1–3 points) groups, respectively (Fig. 3b). Furthermore, the prognostic impact of ANC ≥ 8 × 109/L was limited to JAK2-mutated cases (p = 0.03).
Arterial and venous thrombosis
At a median follow-up time of 8.5 years (range, 0.01–52.7), major thrombosis after diagnosis was documented in 162 (16%) of patients including 127 (13%) arterial and 70 (7%) venous events. Incidence rates of major arterial/venous thrombosis for JAK2, type 1/type 1-like CALR, type 2/type 2-like CALR, MPL-mutated and TN cases were 14%/8%, 11%/6%, 12%/9%, 13%/0%, and 6%/2%, respectively. Figure 4a. illustrates arterial TFS stratified by driver mutations and discloses higher rates in JAK-mutated cases when compared with TN or type 1/type 1-like CALR-mutated (p = 0.02 and 0.12, respectively, HR 3.0, 95% CI 1.2–7.5 and HR 1.5, 95% CI 0.9–2.6). Arterial thrombosis rate was found to be similar in JAK2 and MPL-mutated cases (p = 1.0); on the other hand, a non-significantly higher rate was observed in type 1/type 1-like CALR and type 2/type 2-like CALR-mutated patients in comparison with TN; p = 0.18 and p = 0.12, respectively, HR 1.9, 95% CI 0.7–5.4 and HR 2.3, 95% CI 0.8–6.4 (Table 4). Findings were unchanged when analysis accounted for age and gender differences among the driver mutation categories (Table 4). In addition, univariate analysis identified age ≥60 years, male gender, leukocyte count >11 × 109/l, hypertension, and arterial thrombosis history as predictors of inferior arterial TFS. Multivariable analysis confirmed age ≥60 years (p = 0.001), male gender (p = 0.01), JAK2 mutational status (p = 0.01), hypertension (p = 0.01), and arterial thrombosis history (p = 0.02) as independent predictors of future arterial thrombotic events (Table 2).
A separate analysis of venous TFS also disclosed higher risk in JAK2 and MPL-mutated patients, when compared with TN and type 1/type 1-like CALR-mutated (p = 0.03 and 0.14, respectively, HR 4.9, 95% CI 1.2–20.3 and HR 1.7, 95% CI 0.8–3.5) (Fig. 3b); additionally, we observed a trend for higher risk of venous thrombosis among type 1/type 1-like CALR and type 2/type 2-like CALR-mutated when compared with TN cases; p = 0.17 and p = 0.08, respectively, HR 2.9, 95% CI 0.6–13.3 and HR 3.9, 95% CI 0.8–17.9 (Table 4). Multivariable analysis inclusive of age, gender, venous thrombosis history, and driver mutation category, identified male gender, and venous thrombosis history as independent predictors of venous thrombosis (p = 0.02/< 0.01) (Table 2).
Aspirin therapy appeared to mitigate both arterial and venous thrombosis with arterial and venous thrombosis rates of 5% vs 16% and 3% vs 9% in patients receiving or not receiving aspirin (p < 0.01 and p = 0.02, respectively, HR 0.4, 95% CI 0.2–0.8 and HR 0.4, 95% CI 0.2–0.9). Additional analyses revealed that the apparent differences in arterial and venous thrombosis observed among type 1/type 1-like CALR-mutated and TN cases, were fully accounted for by aspirin use (p value adjusted for aspirin use = 0.40/0.31). Cytoreductive therapy, on the other hand, did not appear to have a clear beneficial impact on neither arterial (p = 0.08) nor venous thrombosis (p = 0.19).
Major hemorrhage
A total of 107 major hemorrhagic events were recorded in 983 patients (11%); 33 of 108 (31%) evaluable patients (including 7 of 22 (32%) with major hemorrhage) had laboratory evidence of acquired von Willebrand syndrome. Incidence rates of major hemorrhage were 12%, 8%, 10%, 20%, and 9%, for JAK2, type 1/type 1-like CALR, type 2/type 2-like CALR, MPL-mutated and TN cases, respectively, with higher rates among MPL-mutated cases compared to type 1/type 1-like CALR, type 2/type 2-like CALR and TN (p = 0.02/0.06/0.02) (Table 4). Additionally, a higher incidence of major hemorrhage was also observed in JAK2-mutated patients compared to type 1/type 1-like CALR-mutated (p = 0.08, HR 1.7, 95% CI 0.9–3.2). Furthermore, on univariate analysis, age ≥60 years (p < 0.01), leukocyte count >11 × 109/l (p < 0.01), presence of cardiovascular risk factors (p < 0.01), and history of major hemorrhage (p = 0.01) predicted future hemorrhage, while aspirin use was of borderline significance (p = 0.10). On multivariable analysis, age ≥60 years (p < 0.01), leukocyte count >11 × 109/l (p < 0.01), and presence of cardiovascular risk factors (p = 0.05) remained significant predictors of major hemorrhage after diagnosis.
Discussion
The current study constitutes the largest single-center series of ET patients who are fully annotated for driver mutations and includes mature survival data and detailed analysis of prognostic factors for overall, leukemia-free, myelofibrosis-free, and thrombosis-free survival, with the latter stratified into arterial vs. venous events. Our study provides baseline clinical and laboratory data and confirms previously recognized differences in age and gender distribution as well as hemoglobin, leukocyte, and platelet levels, among specific driver mutation categories; CALR-mutated and TN patients were younger at diagnosis (median age 52 and 50 years, respectively), compared to JAK2 or MPL-mutated cases (median age 71 and 66 years, respectively); JAK2 and TN patients were predominantly female, compared to CALR and MPL-mutated cases [19]. Noteworthy laboratory associations included higher hemoglobin and leukocyte count with JAK2 mutation and higher platelet count with CALR mutation (type-2 more than type-1) and TN [19]. At the time of diagnosis, approximately 22% of patients displayed history of major arterial (14%) or venous (10%) thrombosis, 8% major hemorrhage, and 29%, microvascular symptoms; incidences of major thrombosis and hemorrhage were lower in CALR-mutated cases [20].
A major strength of the current study was the availability of long-term follow-up data, which enabled accurate estimation of survival and disease transformation rates; median overall survival was 20.6 years, with 10-year/20-year leukemic transformation and fibrotic progression rates of 1.5%/7.6% and 8%/26%, respectively. As previously noted [9], JAK2/CALR/MPL/TN mutational status did not appear to impact overall or leukemia-free survival in our current ET patient cohort while MPL mutation was associated with a significantly higher rate of progression to myelofibrosis, as per previous reports [21, 22]. Prominent risk factors for survival in the current ET patient cohort included older age, ANC ≥ 8 × 109/L, ALC < 1.7 × 109/L, male gender, hypertension, and arterial thrombosis history. These observations are in line with those previously communicated [10, 23]. Application of the recently introduced AAA survival model in ET [10], to the current patient cohort resulted in median survival estimates not reached for low (10-/20-year survival rate 98%/80%), 21.3 years for intermediate-1 (10-/20-year survival rate 93%/60%), and 10.6 years for high/intermediate-2 (10-/20-year survival rate 54%/7%) risk patients (Fig. 1).
Additional observations from the current study are highlighted by (i) the extremely low incidence of leukemic transformation in the absence of abnormal karyotype and ExT (10-/20-year rate of 1.5%/3%), and (ii) the relatively high rate of fibrotic progression in MPL-mutated patients or those with ANC ≥ 8 × 10(9)/L. It is to be recalled that we have previously reported an association between ExT and inferior overall and leukemia-free survival in young (age <40 years) ET patients [24]. However, the association between ExT and leukemic progression in the current study appeared to be limited to patients with JAK2 mutation; this is a noteworthy observation, given that ExT is typically associated with CALR (type-2 more than type-1) and TN mutational status. Interestingly, none of our 84 TN patients with ET experienced leukemic progression. These findings require external validation. The prognostic impact of ANC ≥ 8 × 109/L on MFFS, has not been previously described, and was limited to JAK-mutated cases. Furthermore, in line with prior reports, type 1/type 1-like CALR mutated compared to JAK2-mutated, and TN cases were noted to have a significantly higher risk of fibrotic progression [22]. The current study did not include information on other mutations that have previously been shown to adversely affect survival, including SF3B1, SRSF2 U2AF1 TP53 mutations [12].
The current study also confirms the higher rates of arterial and venous thrombosis displayed by JAK2/MPL-mutated patients with ET, compared with type 1/type 1-like CALR-mutated and TN, and that this effect was not accounted for by other independent risk factors for thrombosis including age, gender, thrombosis history or cardiovascular risk factors [20]. Moreover, a non-significantly higher risk of thrombosis was also observed in CALR-mutated patients in comparison with TN cases; which was no longer apparent after accounting for aspirin use. Together, our findings not only confirm differential thrombosis risk according to driver mutation, but also identify TN patients to have a lower risk of thrombosis, akin to those with CALR mutation. Our observations corroborate the salutary effects of aspirin with respect to both arterial and venous thrombosis, and also suggest possible benefits from aspirin prophylaxis in “very low risk” CALR-mutated patients.
From a practical standpoint, the prognostic information discussed in the current report, regarding survival is not necessarily actionable, since drug therapy in ET has not been shown to be disease-modifying. However, identification of prognostic factors for survival and disease progression is useful for purposes of patient counseling and disease monitoring. On the other hand, risk factor assessment for thrombosis is critical for both primary and secondary prevention measures. In this regard, the observations from the current study underscore the therapeutic value of aspirin for the prevention of both arterial and venous thrombosis. Controlled studies are always preferred over retrospective studies for accurate determination of the optimal therapeutic approach to ameliorate the risk associated with thrombosis and disease progression in ET. In the meantime, we hope the information contained in the current document serves as a complimentary resource for patients and physicians and provides context for the design and interpretation of future clinical trials [12, 25,26,27,28,29].
References
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemia: integrating morphological, clinical, and genomic data. Blood. 2022;140:1200–28.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:1599–613.
Tefferi A, Pardanani A. Essential thrombocythemia. N. Engl J Med. 2019;381:2135–44.
Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857–9.
Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015;5:e366.
Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, et al. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia. 2012;26:716–9.
Szuber N, Mudireddy M, Nicolosi M, Penna D, Vallapureddy RR, Lasho TL, et al. 3023 Mayo Clinic patients with myeloproliferative neoplasms: risk-stratified comparison of survival and outcomes data among disease subgroups. Mayo Clin Proc. 2019;94:599–610.
Szuber N, Vallapureddy RR, Penna D, Lasho TL, Finke C, Hanson CA, et al. Myeloproliferative neoplasms in the young: Mayo Clinic experience with 361 patients age 40 years or younger. Am J Hematol. 2018;93:1474–84.
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–13.
Tefferi A, Loscocco GG, Farrukh F, Szuber N, Mannelli F, Pardanani A, et al. A globally applicable “triple A” risk model for essential thrombocythemia based on age, absolute neutrophil count, and absolute lymphocyte count. Am J Hematol. 2023;98:1829–37.
Gangat N, Jadoon Y, Szuber N, Hanson CA, Wolanskyj-Spinner AP, Ketterling RP, et al. Cytogenetic abnormalities in essential thrombocythemia: clinical and molecular correlates and prognostic relevance in 809 informative cases. Blood Cancer J. 2022;12:44.
Tefferi A, Guglielmelli P, Lasho TL, Coltro G, Finke CM, Loscocco GG, et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol. 2020;189:291–302.
Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369.
Haider M, Gangat N, Lasho T, Abou Hussein AK, Elala YC, Hanson C, et al. Validation of the revised international prognostic score of thrombosis for essential thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic patients. Am J Hematol. 2016;91:390–4.
Tefferi A, Lasho TL, Jimma T, Finke CM, Gangat N, Vaidya R, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc. 2012;87:25–33.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Barbui T, Thiele J, Gisslinger H, Finazzi G, Carobbio A, Rumi E, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52–4.
Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–84.
Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89:E121–124.
Elala YC, Lasho TL, Gangat N, Finke C, Barraco D, Haider M, et al. Calreticulin variant stratified driver mutational status and prognosis in essential thrombocythemia. Am J Hematol. 2016;91:503–6.
Haider M, Elala YC, Gangat N, Hanson CA, Tefferi A. MPL mutations and palpable splenomegaly are independent risk factors for fibrotic progression in essential thrombocythemia. Blood Cancer J. 2016;6:e487.
Loscocco GG, Guglielmelli P, Gangat N, Rossi E, Mannarelli C, Betti S, et al. Clinical and molecular predictors of fibrotic progression in essential thrombocythemia: a multicenter study involving 1607 patients. Am J Hematol. 2021;96:1472–80.
Tefferi A, Betti S, Barraco D, Mudireddy M, Shah S, Hanson CA, et al. Gender and survival in essential thrombocythemia: a two-center study of 1494 patients. Am J Hematol. 2017;92:1193–7.
Gangat N, Szuber N, Jawaid T, Hanson CA, Pardanani A, Tefferi A. Young platelet millionaires with essential thrombocythemia. Am J Hematol. 2021;96:E93–5.
Gangat N, Singh A, Szuber N, Begna K, Elliott M, Wolanskyj-Spinner A, et al. Site-specific venous thrombosis in essential thrombocythemia: Impact on subsequent vascular events and survival. J Thromb Haemost. 2022;20:2439–43.
Gangat N, Szuber N, Jadoon Y, Farrukh F, Begna K, Elliott MA, et al. 1.5 million platelet count limit at essential thrombocythemia diagnosis: correlations and relevance to vascular events. Blood Adv. 2022;6:3835–39.
Karrar OS, Abdelmagid M, Vannucchi AM, Barbui T, Tefferi A, Gangat N. ABO blood group type and risk of venous thrombosis in essential thrombocythemia. Br J Haematol. 2023;202:699–703.
Guglielmelli P, Gangat N, Coltro G, Lasho TL, Loscocco GG, Finke CM, et al. Mutations and thrombosis in essential thrombocythemia. Blood Cancer J. 2021;11:77.
Tefferi A, Lasho TL, Guglielmelli P, Finke CM, Rotunno G, Elala Y, et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016;1:21–30.
Author information
Authors and Affiliations
Contributions
NG and AT designed the study, performed analysis, and co-wrote the paper. OK collected data. AA, KHB, ME, APW, AP contributed patients. CAH reviewed bone marrow morphology. RPK reviewed cytogenetic studies. All authors reviewed and approved the final draft of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gangat, N., Karrar, O., Al-Kali, A. et al. One thousand patients with essential thrombocythemia: the Mayo Clinic experience. Blood Cancer J. 14, 11 (2024). https://doi.org/10.1038/s41408-023-00972-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00972-x
This article is cited by
-
Prediction models for essential thrombocythemia from two longitudinal studies involving 2000 patients
Blood Cancer Journal (2024)
-
Triple a score (AAA: age, absolute neutrophil count and absolute lymphocyte count) and its prognostic utility in patients with overt fibrotic and prefibrotic myelofibrosis
Annals of Hematology (2024)