Abstract
This retrospective study evaluated 35 children (median age 5.2 years; range 0.4–18) with myelofibrosis (MF), including 33 with primary myelofibrosis and 2 with secondary myelofibrosis transplanted from matched sibling donor (MSD) (n = 17) or non-MSD (n = 18) between 2000 and 2022. Conditioning was usually chemotherapy-based (n = 33) and myeloablative (n = 32). Fifteen patients received bone marrow (BM), 14 haematopoietic cells (HC) from peripheral blood (PB), and 6 from cord blood (CB). Day +100 acute GvHD II–IV incidence was significantly lower after MSD-haematopoietic cell transplantation (MSD-HCT) than after non-MSD-HCT [18.8% (4.3–41.1) vs 58.8% (31–78.6); p = 0.01]. Six-year non-relapse mortality (NRM) was 18% (7.1–32.8), relapse incidence was 15.9% (5.6–30.9), progression-free survival (PFS) was 66.1% (47–79.7), GvHD-free relapse-free survival was 50% (30.6–66.7), and overall survival (OS) was 71.1% (51.4–84). Six-year PFS and OS were significantly higher after BM transplantation compared to HCT from other sources [85.1% (52.3–96.1) vs 50.8% (26.3–71), p = 0.03, and 90.9% (50.8–98.7) vs 54% (28.1–74.2), p = 0.01, respectively], whereas NRM was significantly lower [0% vs 32% (12.3–53.9); p = 0.02]. This first multicentre study on outcomes of allogeneic HCT in children with myelofibrosis proves feasibility and curative effect of transplantation in these children, suggests that bone marrow transplantation is associated with better outcomes, and indicates the need for further studies.
Similar content being viewed by others
Introduction
In contrast to the adult population, the classical BCR::ABL1-negative myeloproliferative neoplasms (BCR::ABL1-neg MPNs), i.e. polycythaemia vera (PV), essential thrombocythaemia (ET) and primary myelofibrosis (PMF) [1] are very rare in the paediatric population with an incidence approximately 100×lower than in adults [2].
The discovery of three driver mutated genes JAK2, CALR and MPL was a major landmark in the understanding of BCR::ABL1-neg MPNs [3]. These three mutations are detected in more than 80% of adult patients and correlate with clinical characteristics, disease-related complications as well as with prognosis and therefore are helpful in treatment stratification [4, 5]. However, in children the mentioned driver mutations occur in less than 50% of them, posing a significant diagnostic challenge given the lack of an objective clonal marker [6, 7]. In addition, the thrombocytosis and erythrocytosis not related to clonal myeloproliferation are observed much more frequently in the paediatric population than in adults. Apart from this, in children the normal range of haemoglobin level, haematocrit value and erythrocytes number depend on the child’s age. Thus, in children and adolescents the utility of the WHO diagnostic criteria of ET, PV, and PMF is limited [1]. Therefore, in the majority of paediatric patients the diagnosis of BCR::ABL1-neg MPN is still difficult [8, 9].
As a consequence of the above mentioned factors, the opportunities to perform prospective clinical trials concerning BCR::ABL1-neg MPNs are severely limited in the paediatric population, and in contrast to adults, there is still a lack of established prognostic criteria and treatment recommendations based on these criteria, including specific recommendations for allogeneic haematopoietic cell transplantation (allo-HCT) in paediatric patients suffering from BCR::ABL1-neg MPNs [7, 9].
In adults PMF and post-ET/PV myelofibrosis (post-ET/PV MF) are BCR::ABL1-neg MPNs with the worst survival rates, but allo-HCT can cure a substantial number of patients, especially those younger than 70 years and with a median survival expectation of less than 5 years [10].
In children and adolescents myelofibrosis (MF) is the rarest type of BCR::ABL1-neg MPNs [2, 6, 7], and so far there was no study analysing the outcomes of allo-HCT in a larger group of paediatric patients with MF, and therefore the data on allo-HCT outcomes in them remain casuistic and scant [11,12,13,14].
For this reason this retrospective, multicentre study on transplant-specific characteristics and outcomes of allo-HCT performed in paediatric patients with PMF or post-ET/PV MF between 2000 and 2022 and reported to the European Society for Blood and Marrow Transplantation (EBMT) Registry was carried out within the EBMT Paediatric Diseases Working Party.
Methods
This was a retrospective EBMT Registry-based analysis approved by the EBMT Pediatric Diseases Working Party and performed in a cohort of children and adolescents ( < 18 years) receiving the first allogeneic haematopoietic cell transplantation (allo-HCT) for primary myelofibrosis (PMF), post-essential thrombocythaemia or post-polycythaemia vera secondary myelofibrosis (post-ET/PV MF), and transplanted between January 1st 2000 and December 31st 2022.
The following outcome measures were analysed in the studied cohort of paediatric patients: cumulative incidence of neutrophil recovery defined as an absolute granulocyte count (ANC) greater than 0.5 × 109/l for three consecutive days unsupported by granulocyte colony stimulating factor; platelets recovery defined as a platelet count above 20 ×109/l for three consecutive days (with no platelet transfusions seven days prior) [15, 16]; acute graft-versus-host disease (aGvHD) and chronic GvHD (cGvHD) occurrence graded according to standard criteria [17, 18]; non-relapse mortality (NRM) defined as death without evidence of relapse or progression; relapse incidence (RI) defined as the time from transplantation to first occurrence of relapse or progression; progression-free survival (PFS) defined as the time from transplantation to relapse or death due to neoplasm; GvHD-free relapse-free survival (GRFS) defined as the time from transplantation to first event of aGvHD grade III-IV, extensive cGvHD, relapse or death, and overall survival (OS) defined as the time from transplantation to death from any cause.
Statistical methods
Quantitative variables were described as median, quartile 1 and 3, minimum and maximum. Differences between two groups and quantitative variables were tested using the Wilcoxon test. Qualitative variables were described as number and percentage. Differences between groups and qualitative variables were tested using the Chi-squared test or the Fisher Exact test when Chi-squared test validation was not respected.
OS, PFS, and GRFS were estimated using the Kaplan-Meier method. All outcomes with competing events were estimated using the cumulative incidence function. NRM and RI were mutually competing events. Death and relapse were competing events for GvHD outcomes. Death was a competing event for ANC and platelet recovery, and secondary HCT. Second HCT was a competing event for ANC and platelet recovery. All outcomes were censored at last follow-up. The median follow-up was estimated using the reverse Kaplan-Meier method. Univariable tests of the covariate impact on outcomes were performed using the log-rank test for OS, PFS and GRFS, and Grey’s test for cumulative incidence outcomes. All tests were significant at the level of 0.05 and two-sided. Analyses were performed using the R software version 4.0.2.
Ethical statement
The study was conducted in accordance with the EBMT Guidelines for Retrospective Studies and the principles of the Declaration of Helsinki. EBMT Centres commit to obtain informed consent with the local regulations applicable at the time of transplantation in order to report pseudoanonymised data to the EBMT.
Results
Patient descriptive analysis
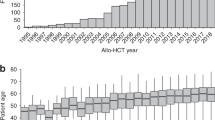
A total of 35 children and adolescents who underwent an allo-HCT for myelofibrosis (MF) between 2000 and 2022 were analysed based on data reported to the EBMT Registry (Table 1). PMF was diagnosed in 33 (94.3%) patients and post-ET/PV MF in two (5.7%). There were 12 (34.3%) female patients and 23 (65.7%) male patients. Median age at diagnosis was 3.4 years (range: 0.1–17.7), while at allo-HCT 5.2 years (range 0.4–18). Median time from diagnosis to transplantation was 7.1 months (range: 3.8–136.4). At transplantation the Lansky score was found to be below 90 in 8 of 32 (25%) patients. Before the start of the conditioning regimen the median haemoglobin level was 9.0 g/dl (range: 5.9–11.8), leucocytes count 6.6 × 109/l (range: 1.1–25.6), platelets count 83 × 109/l (range: 1–1505), and 11 (47.8%) patients had palpable splenomegaly. Splenectomy prior to the start of the preparative regimen was performed in only one (3.2%) patient.
Seventeen (48.6%) children were transplanted from matched sibling donors (MSD), all of them (100%) for PMF, and the remaining 18 (51.4%) underwent transplantation from non-MSD, including 16 (88.9%) for PMF and two (11.1%) for post-ET/PV MF (Table 1). The median age of the children transplanted from MSD was 4.3 years (range: 0.1–17.7) at diagnosis and 4.7 years (range: 0.4–18) at transplantation. Among the children transplanted from non-MSD the median age at diagnosis was 3.2 years (range: 0.3–16.9), while the median age at transplantation was 5.4 years (range: 0.9–17.7). The Lansky score was found to be below 90 in 4 of 16 (25%) recipients transplanted from MSD, and in 4 of 16 (25%) transplanted from non-MSD.
Sixteen (45.7%) patients were transplanted between 2000 and 2007, and the remaining 19 (54.3%) between 2008 and 2022 (Table 1). All 16 (100%) patients transplanted between 2000 and 2008 underwent HCT for PMF, while out of 19 patients who received HCT between 2008 and 2022 seventeen (89.5%) underwent transplantation for PMF and 2 (10.5%) for post-ET/PV MF. The median age at diagnosis and at transplantation in children transplanted between 2000 and 2007 was 3.1 years (range: 0.1–17.4) and 6.0 years (range: 0.4–17.6), respectively, and in those who underwent transplantation between 2008–2022 it was 3.4 years (0.4–17.7) and 5.2 years (range: 0.9–18), respectively. Pre-transplant Lansky performance status below 90 was found in 4 of 14 (28.6%) patients receiving HCT between 2000 and 2007, and in 4 of 18 (22.2%) patients transplanted between 2008 and 2022 (Table 1).
Transplant descriptive analysis
Donor and source of haematopoietic cells (HCs)
Seventeen (48.6%) children were transplanted from MSD, and 18 (51.4%) from non-MSD including three (16.7%) from another matched relative donor, three (16.7%) from a haploidentical donor (including one haploidentical cord blood), and 12 (66.7%) from an unrelated donor (UD) including four cord blood and one double cord blood (Table 2). Eight (22.9%) male recipients received hematopoietic cells (HCs) from female donor, including three (17.6%) female MSD and five (27.8%) female non-MSD.
Eleven (64.7%) patients transplanted from MSD and four (22.2%) transplanted from non-MSD (p = 0.006) received bone marrow (BM), while peripheral blood (PB) was the source of HCs in six (35.3%) recipients undergoing MSD-HCT and in eight (44.4%) recipients undergoing transplantation from non-MSD (Table 2). A further six (33.3%) patients transplanted from non-MSD obtained HCs from cord blood (CB).
Conditioning regimen
Thirty-one (91.2%) patients received myeloablative conditioning (MAC), and only three (8.8%) obtained non-myeloablative, reduced intensity conditioning (RIC) (two transplanted from MSD, and one from non-MSD) (Table 2). The conditioning regimen was chemotherapy-based in 33 (94.3%) patients, while FTBI-based was used only in two (5.7%) transplanted from non-MSD, including one patient obtaining FTBI along with busulfan, cyclophosphamide and fludarabine and one obtaining FTBI alone. A busulfan-based regimen was utilized in 24 (68.6%) patients, including 15 (62.5%) receiving HCs from MSD and 9 (37.5%) transplanted from non-MSD. A treosulfan-based regimen was given exclusively to six (17.1%) recipients who underwent non-MSD HSCT.
Among the 16 patients transplanted between 2000 and 2007 a busulfan-based regimen was administered in 13 (81.2%) patients, fludarabine plus melphalan (FluMel) in two (12.5%) and total body irradiation (TBI) in one (6.3%), while in the 19 patients transplanted between 2008 and 2022 busulfan-based regimen was given in 11 (57.9%), treosulfan-based in 6 (31.5%), cyclophosphamide plus fludarabine (CyFlu) in one (5.3%), and TBI+BuCyFlu in one (5.3%) (Table 2).
GvHD prophylaxis and grading
Data on GvHD prophylaxis were available in 33 children out of 35 (Table 2). In 30 (91.8%) patients GvHD prevention was based on cyclosporin A (CsA). CsA along with short-course methotrexate (MTX) was administered in 19 (57.6%) recipients, including 13 (81.2%) who underwent MSD-HCT and in six (35.3%) who underwent non-MSD-HCT. CsA alone was given in six (18.2%) patients, all of which were transplanted from non-MSD (35.3%). In four (12.1%) recipients CsA was combined with mycophenolate mofetil (MMF), including one (6.2%) who underwent MSD-HCT and in three (16.8%) who underwent non-MSD HSCT. In vivo T-cell depletion (TCD) was performed in 24 (71.7%) patients, among them in 9 (56.2%) before transplantation from MSD and in 15 (88.2%) before transplantation from non-MSD. Complete and detailed data on GvHD prophylaxis for the whole study cohort and according to donor type are presented in Table 2.
Transplant specific outcomes
Neutrophil and platelet engraftment
The day +30 and day +60 cumulative incidence (CI) of neutrophil recovery were 77.1% (58.6–88.1) and 85.7% (67.1–94.2), respectively (Table 3). The day +60 incidence of platelet recovery was 78.1% (58.4–89.3), and after 180 days it was 84.4% (64.7–93.6).
Acute and chronic GvHD
The day +100 CI of aGvHD II–IV was 39.4% (22.7–55.7), and was lower after undergoing MSD-HSCT (18.8%; 4.3–41.1) compared to non-MSD-HSCT (58.8%; 31–78.6) (p = 0.01). The day +100 CI of aGvHD III–IV was 9.1% (2.3–21.9) and was lower (0%) after undergoing MSD-HSCT than observed after undergoing non-MSD transplantation (17.6%; 4.1–39), however, the difference was not significant (p = 0.08) (Table 4).
The 6-year CI of cGvHD was 16.7% (5.9–32.4), including extensive cGvHD 14.6% (4.2–31.2), which at six years from transplantation did not significantly differ in the children transplanted from MSD (6.7%; 0.4–27.1) and from non-MSD (21.9%; 4.4–47.8) (p = 0.35) (Table 4).
Regarding the haematopoietic cells source, i.e. bone marrow vs other sources, there was no significant difference in terms of the day +100 incidence of aGvHD II-IV (42.9%; 16.6–67 vs 36.8%; 15.9–58.2) (p = 0.88) or aGvHD III–IV (7.1%; 0.4–28.5 vs 10.5%; 1.7–29.1) (p = 0.72). The 6-year incidence of cGvHD (22.1%; 4.8–47.1 vs 12.3%; 1.8–33.7) (p = 0.39) and extensive cGvHD (14.3%; 2.1–37.5 vs 13.3%; 1.8–36.3) (p = 0.66) were not significantly different (Table 4).
Non-relapse mortality (NRM)
Six patients died without evidence of relapse, including three patients due to graft failure (13, 32, and 644 days after HCT) and three due to acute GvHD (62, 102, and 109 days after HCT) (Table 5).
In the whole study cohort the 6-year CI of NRM was 17% (7.1–32.8), while in the children transplanted from MSD it was 12.6% (1.9–34.1), which did not differ significantly from those transplanted from non-MSD (22.2%; 6.6–43.6) (p = 0.42), but it was significantly lower in the children obtaining bone marrow (0%) than in those transplanted with hematopoietic stem cells from other sources (32%; 12.3–53.9) (p = 0.02) (Table 6, Fig. 1). The 6-year NRM between 2000 and 2007 (25%; 7.2–48.1) was not significantly different from observed between 2008 and 2022 (10.5%; 1.7–29) (p = 0.37) (Table 6, Fig. 1).
Relapse incidence (RI), progression-free survival (PFS), GvHD-free relapse-free survival (GRFS), and overall survival (OS)
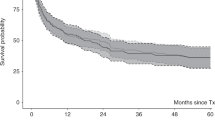
For the whole study group the 6-year RI was 15.9% (7.75.6–30.9), the 6-year PFS was 66.1% (47–79.7), the 6-year GRFS was 50% (30.6–66.7), and the OS was 71.1% (51.4–84) (Table 6, Fig. 2) with a median follow-up of 9.1 years (3.1–11.2).
In univariate analysis, the 6-year RI, PFS, GRFS, and OS probability in children transplanted from MSD in comparison with those transplanted from non-MSD were not significantly different (Table 6; Figs. 3, 4, 5, 6), although the 6-year GRFS probability after undergoing MSD-HCT was almost two times higher (67%; 37.9–84.7) than after non-MSD transplantation (34.9%; 12.4–78.1) (p = 0.07).
In relation to the source of the haematopoietic cells, the 6-year PFS in children obtaining bone marrow (85.1%; 52.3–96.1) was significantly higher than in those obtaining haematopoietic cells from other sources (50.8%; 26.3–71) (p = 0.03) (Table 6; Fig. 4), and the 6-year OS in children receiving bone marrow (90.9%; 50.8–98.7) was significantly higher than in children transplanted with haematopoietic cells from other sources (54%; 28.1–74.2) (p = 0.01) (Table 6; Fig. 6). Apart from that, the 6-year GRFS after undergoing a bone marrow transplantation (70.1%; 38.5–87.6) was close to two times higher than that observed for haematopoietic cells transplantation from other sources (36.1%; 14.5–58.4), however, the difference was not significant (p = 0.09).
With regard to the time period, the 6-year RI, PFS, GRFS, and the OS in children transplanted between 2008 and 2022 were more favourable than in those transplanted within the period 2000–2007, but the differences were not significant (Table 6; Figs. 4, 5, 6).
Discussion
Comparing the paediatric population with the population of adults, the BCR::ABL1-neg MPNs are not only much rarer, but also in more than 50% of paediatric patients these neoplasms are triple-negative, i.e., without the JAK2, CALR, or MPL mutations, that drive BCR::ABL1-neg MPNs in the absolute majority of adult patients [6, 7, 9],
The rarity of BCR::ABL1-neg MPNs in children and adolescents severely limits the opportunities to perform clinical studies on these malignancies in paediatric population, and as a consequence, in contrast to the adult population, there are still no established specific diagnostic and prognostic criteria and clear treatment recommendations based on these criteria, including indications for allo-HCT in paediatric patients suffering from BCR::ABL1-neg MPN. For these reasons, there have been no prospective or even retrospective studies on the long-term outcomes of allo-HCT in a representative group of paediatric patients with BCR::ABL1-neg MPNs, including PMF and post-ET/PV MF.
PMF and post-ET/PV MF are the BCR::ABL1-neg MPNs categories with the worst survival rates in adults and can only be cured by allo-HSCT, which can induce molecular remission and resolution of bone marrow fibrosis (10). In the paediatric population PMF and post-ET/PV MF are the rarest category of BCR::ABL1-neg MPNs [5,6,7], and the reports published so far on results of allo-HCT in these patients remain scanty and casuistic [11,12,13,14].
The current retrospective study on allo-HCT outcomes in children and adolescents transplanted for PMF or post-ET/PV MF was conducted on behalf of the EBMT Paediatric Diseases Working Party and based on data collected in the EBMT Registry. To date the studied group is the largest cohort of children and adolescents with myelofibrosis (MF) receiving allo-HCT ever collected and analysed.
Previously, the largest group of children with primary myelofibrosis treated and cured with allo-HCT was published by Hussain et al. [13]. This cohort consisted of eight patients under two years of age diagnosed during infancy. However, five of these patients had parents with close consanguinity, and two of them had a strong family history of infant myelofibrosis with recurrent early childhood deaths, which might suggest a congenital predisposition. Apart from this report there have been several other reports of familial cases of infant myelofibrosis primarily in regions of the world with a high level of consanguinity [11, 19]. All the familial cases presented at a very young age and had poor outcomes without HSCT. Thus, the cohort described by Hussein et al. [13] seems to be a very specific one that does not necessarily reflect the biology and clinics of PMF and post-ET/PV MF in the whole paediatric population.
Although, allo-HCT can cure a substantial proportion (55%) of adult patients with PMF or post-ET/PV MF, but is still not universally applicable due to risk of its severe toxic, immunological and infectious complications, which leads to therapy-related morbidity and mortality [10, 20].
Therefore, to determine the prognosis and indications for allo-HCT in adult patients with myelofibrosis, the risk scores taking into account patient- and disease-specific risk factors are currently being developed, which also include the molecular profile [21,22,23,24]. In contrast, in children and adolescents with myelofibrosis, the specific prognostic factors have not yet been investigated and in consequence, there are not yet any paediatric risk-scores that can be used to determine the indications for allo-HCT in paediatric patients.
In the studied group of paediatric patients transplanted for myelofibrosis, post-ET/PV MF was a rare indication (5.7%) for allo-HCT, and it was a much less common indication for transplantation than reported in adult patients transplanted for myelofibrosis (21.8%) [20].
The majority of the studied paediatric patients with myelofibrosis were male patients (65.7%) and this may indicate that in the paediatric population, myelofibrosis occurs more often in male patients than in female as opposed to the proportions observed between male and female paediatric patients suffering from essential thrombocythaemia [2, 6, 7, 9]. Indeed, the male predominance (63.2%) among 19 paediatric patients with primary myelofibrosis was also observed by DeLario et al. [25] as well as in adults who underwent allo-HCT for myelofibrosis between 1995 and 2018 (62.8%) [20].
Our study group was characterised by a young median age at diagnosis (3.4 years) and at transplantation (5.2 years). DeLario et al. [25] reported even younger median age (14 months) in 19 pediatric patients with primary myelofibrosis. Such a young age of these patients may indicate the congenital nature of myelofibrosis and the need for molecular studies in these patients to identify specific genetic mutations to guide prognosis and treatment, including allogeneic hematopoietic stem cells;
Apart from the young age of the patients, the median time between diagnosis and transplantation was very short (7.1 months), and much shorter than reported in adults (31.1 months) [20]. The short median time between diagnosis and transplantation may be related to the symptoms of fast progression of the disease, but the median blood counts prior to the start of conditioning regimen for transplantation were only moderately reduced, however, their ranges were wide.
The rate of the Lansky performance status below 90 in the studied children was somewhat lower (25%) than in adults (32.5%), in whom it was identified as one of the factors associated with a worse NRM and OS [20].
Half of the patients from the studied cohort were transplanted from MSD and the second half from non-MSD. The number of patients who underwent allo-HCT between 2000 and 2007 was not significantly different from the number of patients transplanted between 2008 and 2022 (16 vs 19; p = 0.73). Thus, there was a unique opportunity to compare allo-HCT outcomes in paediatric patients transplanted for myelofibrosis from MSD and from non-MSD as well as in those transplanted between 2000 and 2007 and between 2008 and 2022.
In the children transplanted from MSD the median age at diagnosis and at transplantation as well as the Lansky score below 90 were not different to those observed in the children transplanted from non-MSD.
Among the differences between these two subpopulations, the median time between diagnosis and transplantation was significantly shorter in the case of MSD-HCT (4.6 months) than in case of non-MSD-HSCT (10.3 months) (p = 0.004), and bone marrow was a significantly more frequent source of haematopoietic cells (HCs) for MSD-HCT (64.7%) than for non-MSD-HCT (22.2%) (p = 0.006). In the case of adults transplanted for myelofibrosis, the peripheral blood was the predominant source of HCs (in total 88.9%) irrespectively of the donor type [20].
A myeloablative conditioning regimen was given to the vast majority of patients (91.2%). Between 2000 and 2007, it was usually a busulfan-based regimen (81.2%) and no patients obtained a treosulfan-based regimen, whereas between 2008 and 2022, around one third of patients (36.4%) received treosulfan-based regimen, but at this point due to the low number of studied patients it was impossible to compare the transplantation outcomes achieved in patients who received a busulfan-based preparative regimen with those who received a treosulfan-based regimen. However, when looking for the optimal conditioning regimen for paediatric patients with MF, it will be important to perform such a comparison in the future, especially in the context of particulary favourable disease-free survival observed after a treosulfan-based regimen in children with myeloid malignancies along with its reduced organ toxicity, and satisfactory myeloablative and immunosuppressive effects [26].
The issue of the optimal conditioning regimen for adult patients with myelofibrosis remains unclear despite the significant experience in the field, however, Murthy et al. [27] recently demonstrated superior outcomes in a retrospective CIBMTR analysis of 872 patients with conditioning consisted of busulfan and fludarabine in both myeloablative and reduced intensity settings.
While discussing the issue of the optimal conditioning regimen for the children with myelofibrosis an attention can be also drawn to the fact that more than half of the studied children (54.3%) were conditioned for transplantation with busulfan combined with cyclophosphamide, while according to the article by Murthy et al. [27] cited above, in adult patients transplanted for myelofibrosis after myeloablative conditioning based on busulfan and cyclophosphamide the engraftment rates were significantly worse and the risk of acute GvHD grade II-IV significantly higher than observed after myeloablative conditioning based on busulfan and fludarabine. Thus, when looking in the future for the optimal conditioning regimen for paediatric patients with MF, it will be important to evaluate the outcomes of allo-HCT in children achieving the treosulfan-based or busulfan-based conditioning regimen containing fludarabine.
Generally, it is thought that the risk of graft failure and poor graft function in patients transplanted for myelofibrosis is significant due to inflammation, elevated proinflammatory cytokines, fibrosis, and often osteosclerosis within the marrow niche along with splenic sequestration [16, 28]. In the studied cohort of children the day +60 neutrophil and platelet engraftment rates were 85.7% and 78.1%, respectively, and these rates were very similar to those reported by Murthy et al. [27] in adult patients transplanted for myelofibrosis after undergoing myeloablative conditioning based on busulfan and cyclophosphamide (87.2% and 83.7%, respectively), which were significantly worse than the rates observed after myeloablative conditioning based on busulfan and fludarabine (95.2% and 86.1%, respectively). Indeed, as mentioned above, more than half of the studied children (54.3%) were conditioned for transplantation with busulfan and cyclophosphamide without fludarabine. It is also worth noting that Murthy et al. [27] did not observe a difference in the engraftment rates between patients receiving a reduced intensity regimen or myeloablative regimen. In our cohort of patients the non-myeloablative, reduced intensity regimen was used only in three (8.6%) of them. In contrast, as many as 56.5–63.1% of adult recipients with myelofibrosis received the reduced intensity conditioning regimen [20, 27].
In patients undergoing allo-HCT for myelofibrosis, a splenomegaly is also considered to have an impact on engraftment and graft function. Therefore, a splenectomy is an option before transplantation, but a high morbidity and mortality related to splenectomy have been reported, and for this reason, splenectomy is not recommended [10, 29, 30]. In the children studied, a palpable spleen was observed prior to the start of conditioning in almost half them (47.8%), but a splenectomy was performed in only one.
GvHD prophylaxis was almost exclusively CsA-based. T-cell depletion in vivo was used in the absolute majority (88.2%) of patients from the non-MSD-HSCT group, but also in more than half (56.2%) of patients transplanted from MSD, and what more CsA along with short-course methotrexate was administered in the majority of patients who underwent MSD-HCT, while only in around one third of patients who underwent non-MSD-HCT.
The overall day +100 incidence of aGvHD grade II–IV was 39.4%, thus somewhat higher than observed in adults transplanted for myelofibrosis (28–35%) [20], which could be related to the conditioning regimen based on busulfan and cyclophosphamide used in more than half of the studied children, because it was found in adults transplanted for myelofibrosis that the risk of aGvHD grade II–IV and grade III–IV was significantly higher in patients who underwent myeloablative conditioning consisting of busulfan and cyclophosphamide (58.9% and 32.6%, respectively) in comparison with those receiving myeloablative conditioning consisting of busulfan and fludarabine (34.4% and 11.9%, respectively) [27]. In our study group, the day +100 incidence of aGvHD grade II–IV was significantly lower after MSD-HCT (18.8%) than after non-MSD-HCT (58.8%) (p = 0.01), and it cannot be ruled out that at least to some extent it could be related to T-cell depletion in vivo and a short-course methotrexate also used in more than half recipients of HCs from MSD.
In the studied cohort of children, the overall 6-year occurrence of cGvHD (16.7%) and extensive cGvHD (14.6%) were several times lower than reported by McLornan et al. [20] in adults transplanted for myelofibrosis. In contrast to the relationship observed by Murthy et al. [27] between the type of conditioning regimen and the risk of severe aGvHD, these authors could not find a significant association between the conditioning regimen used and the incidence of cGvHD in adults.
In the studied group of paediatric patients the incidence of aGvHD III–IV, cGvHD, and extensive cGvHD after undergoing MSD-HCT was lower than observed after undergoing non-MSD-HSCT, but the differences were not significant.
In the analysed cohort, the cumulative incidence of NRM after 6 years was 18%, while McLornan et al. [20] observed a 30% NRM after three years in adults. Graft failure and aGvHD were the exclusive causes of NRM in the studied children, and it speaks also to a need to optimise the conditioning regimen for allo-HCT and GvHD prophylaxis in children with myelofibrosis. In addition, it is worth noting that in the studied paediatric cohort NRM occurred exclusively in children who received HCs from peripheral blood or cord blood. There were no deaths related to infectious complications or conditioning regimen organ toxicity, whereas in reported adults NRM was related to infections and also to GvHD [20].
For the whole study group the 6-year RI was 15.9%, the PFS was 66.1%, the GRFS was 50%, and the OS was 71.1%. For comparison, in the cohort of adults transplanted between 1995 and 2018 for myelofibrosis studied by McLornan et al. [20] the 3-year RI was 21–24%, the RFS was 47–50%, and the OS was 55–60%. In the studied children transplanted with bone marrow, the 6-year PFS and the 6-year OS were significantly higher than in the children who received HCs from other sources, namely 85.1% vs 50.1% (p = 0.03) and 90.9% vs 54% (p = 0.01), respectively. Thus, taking into the consideration significantly lower NRM along with significantly higher PFS and OS in children transplanted for myelofibrosis with bone marrow, it can be concluded that in paediatric patients with myelofibrosis the bone marrow should be the recommended source of HCs for allo-HCT.
Unlike the case of the studied paediatric patients, McLornan et al. [20] did not identify an impact of the HCs source on the survival outcomes in adults transplanted for myelofibrosis between 1995 and 2018.
In contrast to adult patients transplanted for myelofibrosis between 1995 and 2018 [20], comparing the outcomes in the studied children in relation to the transplant period, the 6-year NRM, RI, PFS, GRFS, and OS in the children transplanted between 2008 and 2022 – despite the trend towards improvement – were not significantly better than those observed in children transplanted between 2000 and 2007 indicating a need to improve the transplant procedure used in children with myelofibrosis.
Several limitations of this study can be recognized, including the retrospective nature of the analysis, small size of the studied cohort of paediatric patients, lack of data on pretransplant treatment, lack of data on mutational status, and lack of comprehensive marrow status data at the time of the allo-HCT.
On the other hand, to date, this is the largest and the first one multicentre study on transplant-specific characteristics and outcomes of allo-HCT for myelofibrosis in paediatric patients. The follow-up time is long and the analysis supports the potentially curative role of allo-HCT for myelofibrosis in children and adolescents. In addition, the study identifies problems related to as extremely rare neoplasm as the myelofibrosis in childhood is, especially in the context of allo-HCT. Namely, there is a lack of comprehensive knowledge about molecular biology of paediatric BCR::ABL1-neg MPNs, including myelofibrosis, and therefore, there is a lack of prognostic factors and prognostic-scores, and in consequence there is a lack of clear indications to assure appropriate selection of paediatric patients for allo-HCT.
In conclusion, this first multicenter study on outcomes of allo-HCT in children with myelofibrosis proves feasibility and curative effect of transplantation in these children, suggests that bone marrow transplantation is associated with better outcomes, and indicates the need for further studies to develop the optimal pretransplant, transplant, and posttransplant allo-HSCT procedures as it takes place in adult patients with myelofibrosis [27, 28, 31]. Taking into consideration the extremely rare occurrence of myelofibrosis in the paediatric population, a prospective, randomised clinical trials seem to be unrealistic, but a prospective observational study could be an acceptable, feasible, and effective compromise between a retrospective study and a prospective, randomized clinical trial.
Data availability
Data are available on request to the EBMT PDWP.
References
Arber DA, Orazi A, Hasserjia RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Hofmann I. Myeloproliferative neoplasms in children. J Hematopathol. 2015;8:143–57.
Grinfeld J, Nangalia J, Green AR. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica. 2017;102:7–17.
Vannucchi AM, Gugielmelli P Molecular prognostication in Ph-negative MPNs in 2022. ASH Education Program - Hematology 2022; pp. 225-34.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95:1599–613.
Ianotto J-C, Curio-Garcia N, Lauermanova M, Radia D, Kiladjian J-J, Harrison CN. Characteristics and outcomes of patients with essential throbocythemia or polycythemia vera diagnosed before 20 years of age: a systemic review. Haematologica. 2019;104:11580–1588.
Sobas M, Kiladijan JJ, Beauverd Y, Curto-Garcia N, Sadjadian P, Shih LY, et al. Real-world study of children and young adults with myeloproliferative neoplasms: identifying risks and unmet needs. Blood Adv. 2022;6:5171–83.
Kucine N, Al.-Kawaaz M, Hajje D, Bussel J, Orazi A. Difficulty distinguishing essential thrombocythaemia from polycythaemia vera in children with JAK2 V617F-positive myeloproliferative neoplasms. Br J Haematol. 2019;185:136–42.
Kucine N. Myeloproliferative neoplasms in children, adolescents and young adults. Curr Hematol Malig. 2020;15:141–6.
Kröger N, Chalandon Y Myeloproliferative Neoplasms. In: Varreras E, Dufour C, Mohty M, Kröger N (Eds.). EBMT Handbook – Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer Open, 2019, pp. 569-78.
Domm J, Calder C, Manes B, Crossno C, Correa H, Frangoul H. Unrelated stem cell transplant for infantile idiopathic myelofibrosis. Pediatr Blood Cancer. 2009;52:893–5.
Shaikh F, Naithani R, Kirby-Allen M, Doyle L. Allogeneic cord hematopoietic stem cell transplantation in an infant with primary myelofibrosis. J Pediatr Hematol Oncol. 2012;34:199–201.
Hussein AA, Domm HT, Al-Zaben A, Frangoul H. Allogeneic hematopoietic stem cell transplantation for infants with idiopathic myelofibrosis. Pediatr Transplantation. 2013;17:815–9.
Mitton B, de Oliveira S, Pullarkat ST, Moore TB. Stem cell transplantation in primary myelofibrosis of childhood. J Pediatr Hematol Oncol. 2013;35:e120–e122.
Valcárcel D, Sureda A Graft failure. In: Carreas E, Dufour C, Mohty M, Kröger N (eds). The EBMT Handbook. Springer International Publishing, 2019. Pp 307-13. http://link.springer.com/10.1007/978-3-030-02278-5_41.
McLornan DP, Boluda JCH, Czerw T, Cross N, Deeg HJ, Ditschkowski M, et al. Allogeneic haematopoietic cell transplantation for myelofibrosis: proposed definitions and management strategies for graft failure, poor graft function and relapse: best practice recommendations of the EBMT Chronic Malignancies Working Party. Leukemia. 2021;35:2445–59.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Rosbach HC. Familial infantile myelofibrosis as an autosomal recesseive disorder: preponderance among children from Saudi Arabia. Pediatr Hematol Oncol. 2006;23:453–4.
McLornan D, Eikema DJ, Czerw T, Kröger N, Koster R, Reinhardt HC, et al. Trends in allogeneic haematopoietic cell transplantation for myelofibrosis in Europe between 1995 and 2018: a CMWP of EBMT retrospective analysis. Bone Marrow Transplant. 2021;56:2160–72.
Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, et al. MIPSS70: Mutation-enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36:310–8.
Kröger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–33.
Passamonti F, Giorgino T, Mora B, Guglielmelli P, Rumi E, Maffioli M, et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31:2726–31.
Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassimat B, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133:2233–342.
DeLario MR, Sheehan AM, Ataya R, Bertuch AA, Vega CII, Webb CR, et al. Clinical, histopathologic, and genetic features of pediatric primary myelofibrosis – an entity different from adults. Am J Hematol. 2012;87:461–5.
Wachowiak J, Sykora K-W, Cornish J, Chybicka A, Kowalczyk JR, Gorczyńska E, et al. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT Pediatric Diseases Working Party. Bone Marrow Transplant. 2011;4:1510–8.
Murthy GSG, Kim S, Estrada-Merly N, Abid MB, Aljurf M, Assal A, et al. Association between the choice of the conditioning regimen and outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica. 2023;108:1900–8.
Perram J, Ross DM, McLornan D, Gowin K, Kröger N, Gupta V, et al. Innovative strategies to improve hematopoietic stem cell transplant outcomes in myelofibrosis. Am J Hematol. 2022;97:1464–77.
Tefferi A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN. Splenectomy in myelofibrosis with myeloid metaplasia: a single-institution experience with 223 patients. Blood. 2000;95:2226–33.
Malato A, Rossi E, Tiribelli M, Mendicino F, Pugliese N. Splenectomy in myelofibrosis: indications, efficacy, and complications. Clin Lymphoma Myeloma Leuk. 2020;20:588–95.
Gagelmann N, Kröger N. Improving allogeneic stem cell transplantation in myelofibrosis. Int J Hematol. 2022;115:619–25.
Acknowledgements
We thank the principal investigators, the clinical staff, and the data managers from the hematopoietic stem cell transplant centers participating in the study.
Author information
Authors and Affiliations
Contributions
JW conceived and designed the study; JW, RR, HA, RFW, J-HD, RPdL, PS, AB, TS, IB, MGV, BG, R-MH, KG, KP, AS-S, PS, AU, PA, APM, FR, MA, AI, AT, MB, and SC provided study patients; J-EG and AD, collected and assembled data; J-EG, AD, and JW analysed the data, interpreted the results and created tables and figures; JW and J-EG wrote the manuscript; all authors contributed to the final version of the manuscript and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wachowiak, J., Galimard, JE., Dalissier, A. et al. Outcomes of allogeneic haematopoietic cell transplantation for myelofibrosis in children and adolescents: the retrospective study of the EBMT Paediatric Diseases WP. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02286-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02286-3