Abstract
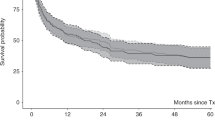
Allogeneic hematopoietic cell transplantation (allo-HCT) remains the only curative option in MF. There is no consensus on the optimal conditioning regimen. We report outcomes of 187 patients with MF transplanted between 2010 and 2017 conditioned with TBF. Median age was 58 years. Median interval from diagnosis to allo-HCT was 44 months. Donors were haploidentical (41%), unrelated (36%) or HLA-identical siblings (23%). Stem cell source was PB in 60%. Conditioning was myeloablative in 48% of cases. Antithymocyte globulin (ATG) was used in 41% of patients. At 100 days, neutrophil and platelet engraftment were 91% and 63% after a median of 21 and 34 days, respectively. Grade II-IV and III-IV acute GVHD occurred in 24% and 12%, while at 3 years, all grade chronic GVHD and chronic extensive GVHD had been diagnosed in 38% and 11%. At 3 years, OS, RFS and GRFS were 55%, 49% and 43%, respectively. RI and NRM were 17% and 33%. On multivariate analysis, poor KPS and the use of unrelated donors were associated with worse GRFS and a higher grade II-IV acute GVHD, respectively. Neither donor type nor intensity of the conditioning regimen influenced survival outcomes. TBF is a feasible conditioning regimen in allo-HCT for MF in all donor settings although longer term outcomes are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International working group for myelofibrosis research and treatment. Blood. 2009;113:2895–901.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Cazzola M, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116:2857–8.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7.
Tefferi A, Guglielmelli P, Lasho TL, Gangat N, Ketterling RP, Pardanani A, et al. MIPSS70+ Version 2.0: mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J Clin Oncol. 2018;36:1769–70.
Harrison C, Kiladjian JJ, Kathrin Al-Ali H, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98.
Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100:1139–45.
Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–91.
Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO, et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Br J Haematol. 2011;152:331–9.
Kröger N, Giorgino T, Scott BL, Ditschkowski M, Alchalby H, Cervantes F, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–50.
Guardiola P, Anderson JE, Gluckman E. Myelofibrosis with myeloid metaplasia. N. Engl J Med 2000;343(Aug):659.
Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16:358–67.
Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009;114:5264–70.
Patriarca F, Bacigalupo A, Sperotto A, Isola M, Bruno B, van Lint MT, et al. Outcome of allogeneic stem cell transplantation following reduced-intensity conditioninig regimen in patients with idiopathic myelofibrosis: the g.I.T.m.o. experience. Mediterr J Hematol Infect Dis. 2010;2:e2010010.
Patriarca F, Bacigalupo A, Sperotto A, Isola M, Soldano F, Bruno B, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica. 2008;93:1514–22.
Sanz J, Boluda JCH, Martín C, González M, Ferrá C, Serrano D, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47:1287–93.
Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849–57.
Shouval R, Vega Y, Fein JA, Danylesko I, Shem Tov N, Yerushalmi R, et al. Allogeneic hematopoietic stem cell transplantation with fludarabine, busulfan, and thiotepa conditioning is associated with favorable outcomes in myelofibrosis. Bone Marrow Transplant. 2020;55:147–56.
Memoli M, Paviglianiti A, Malard F, Battipaglia G, Brissot E, Médiavilla C, et al. Thiotepa-busulfan-fludarabine as a conditioning regimen for patients with myelofibrosis undergoing allogeneic hematopoietic transplantation: a single center experience. Leuk Lymphoma. 2020:1–9, https://doi.org/10.1080/10428194.2020.1827246.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Saraceni F, Beohou E, Labopin M, Arcese W, Bonifazi F, Stepensky P, et al. Thiotepa, busulfan and fludarabine compared to busulfan and cyclophosphamide as conditioning regimen for allogeneic stem cell transplant from matched siblings and unrelated donors for acute myeloid leukemia. Am J Hematol. 2018;93:1211–9.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–1.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 2009;446:496–509.
McLornan DP, Yakoub-Agha I, Robin M, Chalandon Y, Harrison CN, et al. State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica. 2019;104:659–68.
Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassinat B, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133:2233–42.
Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2016;22:1206–11.
McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and reduced-intensity conditioned allogeneic hematopoietic stem cell transplantation in myelofibrosis: a retrospective study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplantant 2019;25:2167–71.
Gowin K, Ballen K, Ahn KW, Hu ZH, Ali H, Arcasoy MO, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4:1965–73.
Raj K, Eikema DJ, McLornan DP, Olavarria E, Blok HJ, Bregante S, et al. Family mismatched allogeneic stem cell transplantation for myelofibrosis: report from the Chronic Malignancies Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:522–8.
Bregante S, Dominietto A, Ghiso A, Raiola AM, Gualandi F, Varaldo R, et al. Improved outcome of alternative donor transplantations in patients with myelofibrosis: from unrelated to haploidentical family donors. Biol Blood Marrow Transplant. 2016;22:324–9.
Robin M, Chevret S, Koster L, Wolschke C, Yakoub-Agha I, Bourhis JH, et al. Antilymphocyte globulin for matched sibling donor transplantation in patients with myelofibrosis. Haematologica. 2019;104:1230–6.
Ciurea S, Champlin RE. Donor selection in T cell—replete haploidentical hematopoietic stem cell transplantation: knowns, unknowns, and controversies. Biol Blood Marrow Transplant. 2013;19:180–4.
Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation. J Hematol Oncol. 2016;9:35.
Battipaglia G, Ruggeri A, Brissot E, Mamez AC, Malard F, Belhocine R, et al. Safety and feasibility of romiplostim treatment for patients with persistent thrombocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50:1574–7.
De Latour RP, Chevret S, Ruggeri AL, Suarez F, Souchet L, Michonneau D, et al. Romiplostim in patients undergoing hematopoietic stem cell transplantation: results of a phase 1/2 multicenter trial. Blood. 2020;135:227–9.
Halahleh K, Gale RP, Da’na W, Ma’koseh M, Saadeh S, Alan W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant. 2020. https://doi.org/10.1038/s41409-020-0975-5.
Shanavas M, Popat U, Michaelis LC, Fauble V, McLornan D, Klisovic R, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to janus kinase 1/2 inhibitors. Biol Blood Marrow Transplant. 2016;22:432–40.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
Author information
Authors and Affiliations
Contributions
GB designed the study and wrote the manuscript. LW, KM and LCDW checked data and performed the statistical analysis. GB, MK, DM,TC, PH and IYA revised the manuscript and all the authors revised its final version. all authors revised the final version. EA, MM, WA, SS, MTR, NK, MLF, DB, API, RF, YC, PP, GM, PC, MS, CS, NC, LCDW, TC, JCHB were the principal investigators at the centres recruiting the highest number of patients for the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Battipaglia, G., Mauff, K., Wendel, L. et al. Thiotepa–busulfan–fludarabine (TBF) conditioning regimen in patients undergoing allogeneic hematopoietic cell transplantation for myelofibrosis: an outcome analysis from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 56, 1593–1602 (2021). https://doi.org/10.1038/s41409-021-01222-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01222-z
This article is cited by
-
Graft-versus-host disease and impact on relapse in myelofibrosis undergoing hematopoietic stem cell transplantation
Bone Marrow Transplantation (2024)
-
The application of JAK inhibitors in the peri-transplantation period of hematopoietic stem cell transplantation for myelofibrosis
Annals of Hematology (2024)
-
Treosulfan compared to busulfan in allogeneic haematopoietic stem cell transplantation for myelofibrosis: a registry-based study from the Chronic Malignancies Working Party of the EBMT
Bone Marrow Transplantation (2024)
-
Improving allogeneic stem cell transplantation in myelofibrosis
International Journal of Hematology (2022)