Abstract
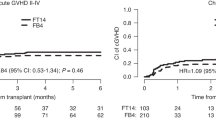
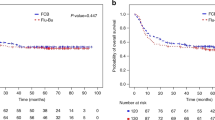
We aimed to compare outcomes following treosulfan (TREO) or busulfan (BU) conditioning in a large cohort of myelofibrosis (MF) patients from the EBMT registry. A total of 530 patients were included; 73 received TREO and 457 BU (BU ≤ 6.4 mg/kg in 134, considered RIC, BU > 6.4 mg/kg in 323 considered higher dose (HD)). Groups were compared using adjusted Cox models. Cumulative incidences of engraftment and acute GVHD were similar across the 3 groups. The TREO group had significantly better OS than BU-HD (HR:0.61, 95% CI: 0.39–0.93) and a trend towards better OS over BU-RIC (HR: 0.66, 95% CI: 0.41–1.05). Moreover, the TREO cohort had a significantly better Progression-Free-Survival (PFS) than both the BU-HD (HR: 0.57, 95% CI: 0.38–0.84) and BU-RIC (HR: 0.60, 95% CI: 0.39–0.91) cohorts, which had similar PFS estimates. Non-relapse mortality (NRM) was reduced in the TREO and BU-RIC cohorts (HR: 0.44, 95% CI: 0.24–0.80 TREO vs BU-HD; HR: 0.54, 95% CI: 0.28–1.04 TREO vs BU-RIC). Of note, relapse risk did not significantly differ across the three groups. In summary, within the limits of a registry-based study, TREO conditioning may improve PFS in MF HSCT and have lower NRM than BU-HD with a similar relapse risk to BU-RIC. Prospective studies are needed to confirm these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data are available on request to the corresponding author.
References
Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36:310–8.
Tefferi A, Guglielmelli P, Lasho TL, Gangat N, Ketterling RP, Pardanani A, et al. MIPSS70+ version 2.0: mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J Clin Oncol. 2018;36:1769–70.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Cazzola M, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116:2857–8.
Tefferi A, Guglielmelli P, Nicolosi M, Mannelli F, Mudireddy M, Bartalucci N, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32:1631–42.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Maffioli M, Mora B, Ball S, Iurlo A, Elli EM, Finazzi MC, et al. A prognostic model to predict survival after 6 months of ruxolitinib in patients with myelofibrosis. Blood Adv. 2022;6:1855–64.
Kröger N. Reduced intensity vs. standard conditioning followed by allogeneic stem cell transplantation for patients with MDS or secondary AML: a prospective, randomized phase III study of the chronic malignancies working party of the EBMT (RICMAC-Trial) [Abstract ASH 2014]. Blood. 2014;124:320.
Kröger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015; https://doi.org/10.1038/leu.2015.233.
McLornan DP, Yakoub-Agha I, Robin M, Chalandon Y, Harrison CN, Kroger N. State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica. 2019;104:659–68.
Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–91.
Robin M, Francois S, Huynh A, Cassinat B, Bay J-O, Cornillon J, et al. Ruxolitinib before allogeneic hematopoietic stem cell transplantation (HSCT) in patients with myelofibrosis: a preliminary descriptive report of the JAK ALLO study, a phase II trial sponsored by goelams-FIM in collaboration with the Sfgmtc. Blood. 2013;122:306.
McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and reduced-intensity conditioned allogeneic hematopoietic stem cell transplantation in myelofibrosis: a retrospective study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:2167–71.
Deeg HJ, Salit RB, Monahan T, Schoch G, McFarland C, Scott BL, et al. Early mixed lymphoid donor/host chimerism is associated with improved transplant outcome in patients with primary or secondary myelofibrosis. Biol Blood Marrow Transplant. 2020;26:2197–203.
Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2016;22:1206–11.
Jain T, Kunze KL, Temkit M, Partain DK, Patnaik MS, Slack JL, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2019;54:204–11.
Popat U, Mehta RS, Bassett R, Kongtim P, Chen J, Alousi AM, et al. Optimizing the conditioning regimen for hematopoietic cell transplant in myelofibrosis: long-term results of a prospective phase II clinical trial. Biol Blood Marrow Transplant. 2020;26:1439–45.
Patriarca F, Masciulli A, Bacigalupo A, Bregante S, Pavoni C, Finazzi MC, et al. Busulfan- or thiotepa-based conditioning in myelofibrosis: a phase II multicenter randomized study from the GITMO group. Biol Blood Marrow Transplant. 2019;25:932–40.
Battipaglia G, Mauff K, Wendel L, Angelucci E, Mohty M, Arcese W, et al. Thiotepa-busulfan-fludarabine (TBF) conditioning regimen in patients undergoing allogeneic hematopoietic cell transplantation for myelofibrosis: an outcome analysis from the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2021;56:1593–602.
Chiusolo P, Bregante S, Giammarco S, Lamparelli T, Casarino L, Dominietto A, et al. Full donor chimerism after allogeneic hematopoietic stem cells transplant for myelofibrosis: the role of the conditioning regimen. Am J Hematol. 2021;96:234–40.
Shouval R, Vega Y, Fein JA, Danylesko I, Shem Tov N, Yerushalmi R, et al. Allogeneic hematopoietic stem cell transplantation with fludarabine, busulfan, and thiotepa conditioning is associated with favorable outcomes in myelofibrosis. Bone Marrow Transplant. 2020;55:147–56.
Claudiani S, Marktel S, Piemontese S, Assanelli A, Lupo-Stanghellini MT, Carrabba M, et al. Treosulfan based reduced toxicity conditioning followed by allogeneic stem cell transplantation in patients with myelofibrosis. Hematol Oncol. 2016;34:154–60.
Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant. 2012;47:1274–82.
Sakellari I, Mallouri D, Gavriilaki E, Batsis I, Kaliou M, Constantinou V, et al. Survival advantage and comparable toxicity in reduced-toxicity treosulfan-based versus reduced-intensity busulfan-based conditioning regimen in myelodysplastic syndrome and acute myeloid leukemia patients after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:445–51.
Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–50.
Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28:3344–51.
Atagunduz IK, Klyuchnikov E, Wolschke C, Janson D, Heidenreich S, Christopeit M, et al. Treosulfan-based conditioning regimen for second allograft in patients with myelofibrosis. Cancers. 2020;12:E3098.
Shimoni A, Robin M, Iacobelli S, Beelen D, Mufti GJ, Ciceri F, et al. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome using treosulfan based compared to other reduced-intensity or myeloablative conditioning regimens. A report of the chronic malignancies working party of the EBMT. Br J Haematol. 2021;195:417–28.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Reményi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108:1497–503.
Iacobelli S.EBMT statistical committeeSuggestions on the use of statistical methodologies in studies of the European Group For Blood And Marrow Transplantation. Bone Marrow Transplant. 2013;48:S1–37.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25.
Tamari R, McLornan DP, Ahn KW, Estrada-Merly N, Hernández-Boluda JC, Giralt S, et al. A simple prognostic system in patients with myelofibrosis undergoing allogeneic stem cell transplantation: a CIBMTR/EBMT analysis. Blood Adv. 2023;7:3993–4002.
Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–70.
Beelen DW, Stelljes M, Reményi P, Wagner-Drouet E-M, Dreger P, Bethge W, et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: final analysis of a prospective randomized trial. Am J Hematol. 2022;97:1023–34.
Gavriilaki E, Labopin M, Sakellari I, Salmenniemi U, Yakoub-Agha I, Potter V, et al. Comparative study of treosulfan plus Fludarabine (FT14) with busulfan plus Fludarabine (FB4) for acute myeloid leukemia in first or second complete remission: an analysis from the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party (ALWP). Bone Marrow Transplant. 2022;57:1803–9.
Malagola M, Polverelli N, Martino M, Patriarca F, Bruno B, Giaccone L, et al. Busulfan or treosulfan conditioning platform for allogeneic stem cell transplantation in patients aged >60 Y with acute myeloid leukemia/myelodysplastic syndrome: a subanalysis of the GITMO AlloEld study. Transplant Direct. 2023;9:e1451.
Murthy GSG, Kim S, Estrada-Merly N, Abid MB, Aljurf M, Assal A, et al. Association between the choice of the conditioning regimen and outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica. 2023;108:1900–8.
Acknowledgements
All participating centers and patients who gave their consent for the registry are thanked.
Author information
Authors and Affiliations
Contributions
MR initiated and proposed the study to the CMWP, MR, TC, NP, JDS, KR, JCHD, and DML were involved in the study design, SI made the statistics, LK made data management, MR, JP, AD, KW, US, PD, PVDB, SR, TCl, SB, JAPS recruited patients and checked the data, all co-authors approved the paper and contributed to the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robin, M., Iacobelli, S., Koster, L. et al. Treosulfan compared to busulfan in allogeneic haematopoietic stem cell transplantation for myelofibrosis: a registry-based study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02269-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02269-4