Abstract
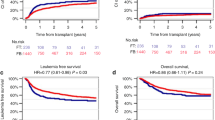
We compared FT14 (fludarabine 150–160 mg/m2, treosulfan 42 g/m2) versus FB4 (fludarabine 150–160 mg/m2, busulfan 12.8 mg/kg) in acute myeloid leukemia (AML) transplanted at primary refractory/relapsed disease. We retrospectively studied: (a) adults diagnosed with AML, (b) recipients of first allogeneic hematopoietic stem cell transplantation (HSCT) from unrelated/sibling donor (2010–2020), (c) HSCT with primary refractory/relapsed disease, (d) conditioning regimen with FT14 or FB4. We studied 346 patients, 113 transplanted with FT14, and 233 with FΒ4. FT14 patients were significantly older, more frequently had an unrelated donor and had received a lower dose of fludarabine. Cumulative incidence (CI) of acute graft-versus-host disease (GVHD) grade III-IV and extensive chronic GVHD was similar. With a median follow-up of 28.7 months, 2-year CI of relapse was 43.4% in FT14 versus 53.2% in FB4, while non-relapse mortality (NRM) was respectively 20.8% versus 22.6%. This led to 2-year leukemia-free survival (LFS) of 35.8% for FT14 versus 24.2% in FB4, and overall survival (OS) of 44.4% versus 34%. Adverse cytogenetics and conditioning regimen independently predicted CI of relapse. Furthermore, conditioning regimen was the only independent predictor of LFS, OS, and GVHD-free/relapse-free survival. Therefore, our real-world multicenter study suggests that FT14 is associated with better outcomes in primary refractory/relapsed AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data will be immediately available upon request to the corresponding author
References
Snowden JA, Sanchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39. https://doi.org/10.1038/s41409-022-01691-w.
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56:1651–64. https://doi.org/10.1038/s41409-021-01227-8.
Poiani M, Labopin M, Battipaglia G, Beelen DW, Tischer J, Finke J, et al. The impact of cytogenetic risk on the outcomes of allogeneic hematopoietic cell transplantation in patients with relapsed/refractory acute myeloid leukemia: On behalf of the acute leukemia working party (ALWP) of the European group for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96:40–50. https://doi.org/10.1002/ajh.26000.
Zhu S, Liu G, Liu J, Chen Q, Wang Z. Long-term outcomes of treosulfan- vs. busulfan-based conditioning regimen for patients with myelodysplastic syndrome and acute myeloid leukemia before hematopoietic cell transplantation: a systematic review and meta-analysis. Front Oncol. 2020;10:591363 https://doi.org/10.3389/fonc.2020.591363.
Sakellari I, Gavriilaki E, Mallouri D, Batsis I, Varelas C, Tagara S, et al. Survival advantage of treosulfan plus fludarabine before allogeneic hematopoietic cell transplantation for older or comorbid patients with myeloid malignancies. Transpl Cell Ther. 2021;27:916.e911–916. https://doi.org/10.1016/j.jtct.2021.07.020.
Gavriilaki E, Sakellari I, Labopin M, Salmenniemi U, Yakoub-Agha I, Potter V, et al. Comparative study of treosulfan plus fludarabine (FT14) with busulfan plus fludarabine (FB4) for acute myeloid leukemia in first or second complete remission: an analysis from the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party (ALWP). Blood. 2021;138:1787–1787. https://doi.org/10.1182/blood-2021-146303
Shimoni A, Labopin M, Savani B, Hamladji RM, Beelen D, Mufti G, et al. Intravenous busulfan compared with treosulfan-based conditioning for allogeneic stem cell transplantation in acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:751–7. https://doi.org/10.1016/j.bbmt.2017.12.776.
Saraceni F, Labopin M, Brecht A, Kroger N, Eder M, Tischer J, et al. Fludarabine-treosulfan compared to thiotepa-busulfan-fludarabine or FLAMSA as conditioning regimen for patients with primary refractory or relapsed acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2019;12:44 https://doi.org/10.1186/s13045-019-0727-4.
Saraceni F, Scortechini I, Fiorentini A, Dubbini MV, Mancini G, Federici I, et al. Conditioning regimens for frail patients with acute leukemia undergoing allogeneic stem cell transplant: how to strike gently. Clin Hematol Int. 2021;3:153–60. https://doi.org/10.2991/chi.k.210731.001.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020;188:129–46. https://doi.org/10.1111/bjh.16355.
Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol. 2018;5:e161–e169. https://doi.org/10.1016/S2352-3026(18)30022-X.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61. https://doi.org/10.1200/JCO.2016.70.7091.
Rambaldi A, Grassi A, Masciulli A, Boschini C, Mico MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–36. https://doi.org/10.1016/S1470-2045(15)00200-4.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25. https://doi.org/10.1038/s41409-020-0803-y.
Fernando F, Robertson HF, El-Zahab S, Pavlu J, How I. Use measurable residual disease in the clinical management of adult acute lymphoblastic. Leuk Clin Hematol Int. 2021;3:130–41. https://doi.org/10.2991/chi.k.211119.001.
Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32:1787–94. https://doi.org/10.1038/s41375-018-0185-y.
Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant. 2012;47:1274–82. https://doi.org/10.1038/bmt.2012.4.
Jayani RV, Pidala J, Jim H, Whiting J, Mo Q, Mishra A. Association of patient-reported physical activity on allogeneic hematopoietic cell transplant outcomes. Clin Hematol Int. 2021;3:34–9. https://doi.org/10.2991/chi.k.210221.001.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39. https://doi.org/10.1016/S2352-3026(19)30157-7.
Beelen DW, Stelljes M, Remenyi P, Wagner-Drouet EM, Dreger P, Bethge W, et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: final analysis of a prospective randomized trial. Am J Hematol. 2022;97:1023–34. https://doi.org/10.1002/ajh.26620.
Kawalec PH, Bungey P, Moseley G, Żegleń O, Markiewicz M. Treosulfan-based conditioning vs. low-dose busulfan-based conditioning for allogeneic hematopoietic stem cell transplantation: a cost-utility analysis in Poland. Acta Haematol Pol. 2022;53:191–200.
Bungey G, Howard D, Leoncini E. Treosulfan-base conditioning vs busulfan-based conditioning for allogeneic haematopoietic stem cell transplantation in patients with aml or mds — a UK cost-utility analysis. Value Health. 2019;22:241.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic. Cell Transplant Clin Hematol Int. 2020;2:2–4. https://doi.org/10.2991/chi.d.191207.001.
Acknowledgements
EG is supported by the ASH Global Research Award.
Author information
Authors and Affiliations
Contributions
EG and IS designed research and wrote the original draft, provided clinical data; ML participated in research design, performed statistical analysis, and drafted the tables and figures; MB, RMH, JC, ME, PZ, IYA, FC, TS, TZ, GK, MY, FN, JF, JLDM, AB, IH, MV, AO, AN provided clinical data and edited the manuscript; BS, AS, AN and MM participated in research design and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavriilaki, E., Sakellari, I., Labopin, M. et al. Survival advantage of treosulfan plus fludarabine (FT14) compared to busulfan plus fludarabine (FB4) in active acute myeloid leukemia post allogeneic transplantation: an analysis from the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party (ALWP). Bone Marrow Transplant 58, 1084–1088 (2023). https://doi.org/10.1038/s41409-023-02028-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02028-x