Abstract
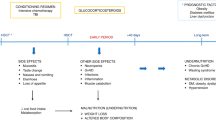
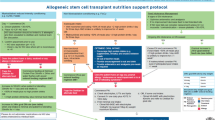
Recommendations on screening and nutritional support for patients undergoing hematopoietic stem cell transplantation (HSCT) have been presented by international nutritional societies, but nutritional practices remain poorly standardized. Following the general policy of the European Society for Blood and Marrow Transplantation (EBMT) to standardize transplantation procedures, the Complications and Quality of Life Working Party and Nursing Research Group carried out a survey among all EBMT centers about their current nutritional practices. The aim of this study was to better understand current practices, differences from available guidelines, and possible barriers for recommended nutritional therapy. Responses from 90 centers (19%) from 23 countries were received. We observed a marked variability in nutritional care between EBMT centers and a substantial lack of standardized operating procedures in screening patients for malnutrition and management of gastrointestinal GVHD. Furthermore, our study confirmed neutropenic diet as standard of care in most centers as well a preference for parenteral nutritional support over enteral. On the basis of these findings, future EBMT efforts will focus on better implementation of international nutritional guidelines into clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K, et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49:1505–12.
Navarro WH, Agovi MA, Logan BR, Ballen K, Bolwell BJ, Fangoul H, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010;16:1442–50.
Le Blanc K, Ringden O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003;88:1044–52.
Deeg HJ, Seidel K, Bruemmer B, Pepe MS, Applebaum FR. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant. 1995;15:461–8.
Walrath M, Bacon C, Foley S, Fung HC. Gastrointestinal side effects and adequacy of enteral intake in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2015;30:305–10.
Iestra JA, Fibbe WE, Zwinderman AH, van Staveren WA, Kromhout D. Parenteral nutrition following intensive cytotoxic therapy: an exploratory study on the need for parenteral nutrition after various treatment approaches for haematological malignancies. Bone Marrow Transplant. 1999;23:933–9.
Urbain P, Birlinger J, Lambert C, Finke J, Bertz H, Biesalski HK. Longitudinal follow-up of nutritional status and its influencing factors in adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:446–51.
Iestra JA, Fibbe WE, Zwinderman AH, van Staveren WA, Kromhout D. Body weight recovery, eating difficulties and compliance with dietary advice in the first year after stem cell transplantation: a prospective study. Bone Marrow Transplant. 2002;29:417–24.
Schulte C, Reinhardt W, Beelen D, Mann K, Schaefer U. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22:1171–8.
ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enter Nutr. 2002;26:1SA–138SA.
August DA, Huhmann MB. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. J Parenter Enter Nutr. 2009;33:472–500.
Bozzetti F, Arends J, Lundholm K, Mickelwright A, Zurcher G, Muscaritoli M, et al. ESPEN Guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009;28:445–54.
van Dalen EC, Mank A, Leclercq E, Mulder RL, Davies M, Kersten MJ, et al. Low bacterial diet versus control diet to prevent infection in cancer patients treated with chemotherapy causing episodes of neutropenia. Cochrane Database Syst Rev. 2016;4:CD006247.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64.
Pizzo PA, Purvis DS, Waters C. Microbiological evaluation of food items for patients undergoing gastrointestinal decontamination and protected isolation. J Am Diet Assoc. 1982;81:272–9.
Baumgartner A, Bargetzi M, Bargetzi A, Zueger N, Medinger M, Passweg J, et al. Nutritional support practices in hematopoietic stem cell transplantation centers: a nationwide comparison. Nutrition. 2017;35:43–50.
Botti S, Liptrott SJ, Gargiulo G, Orlando L. Nutritional support in patients undergoing haematopoietic stem cell transplantation: a multicenter survey of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) transplant programmes. Ecancermedicalscience. 2015;9:545.
Fuji S, Mori T, Lee V, Cheng J, Linton N, Lie A, et al. A multi-center international survey related to the nutritional support after hematopoietic stem cell transplantation endorsed by the ASIA Pacific Blood and Marrow Transplantation (APBMT). Food Nutr Sci. 2012;3:417–4219.
Fuji S, Mori T, Khattry N, Cheng J, Do Y, Yakushijin K, et al. Severe weight loss 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transplant. 2014;50:100–5.
Fuji S, Einsele H, Savani BN, Kapp M. Systematic nutritional support in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2015;21:1707–13.
Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. 2015;34:335–40.
Rzepecki P, Barzal J, Sarosiek T, Oborska S, Szczylik C. Which parameters of nutritional status should we choose for nutritional assessment during hematopoietic stem cell transplantation? Transplant Proc. 2007;39:2902–4.
Urbain P, Birlinger J, Ihorst G, Biesalsi HK, Finke J, Bertz H. Body mass index and bioelectrical impedance phase angle as potentially modifiable nutritional markers are independent risk factors for outcome in allogeneic hematopoietic cell transplantation. Ann Hematol. 2013;92:111–9.
Jubelirer SJ. The benefit of the neutropenic diet: fact or fiction? Oncologist. 2011;16:704–7.
French M. A survey of the use of low mibrobial diets in pediatric transplant programs. J Am Diet Assoc. 2001;101:1194–8.
Trifilio S, Helenowski I, Giel M, Gobel B, Pi J, Greenberg D, et al. Questioning the role of a neutropenic diet following hematopoetic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1385–90.
Baumgartner A, Bargetzi A, Zueger N, Bargetzi M, Medinger M, Bounoure L, et al. Revisiting nutritional support for allogeneic hematologic stem cell transplantation-a systematic review. Bone Marrow Transplant. 2017;52:506–13.
Masszi T, Mank A. Supportive care. In: Apperley J, Carreras E, Gluckman E, Masszi T, editors. EBMT-ESH Handbook. Paris, France: ESH; 2002. p. 156–75.
Fox N, Freifeld A. The neutropenic diet reviewed: Moving toward a safe food handling approach. Oncology. 2012;26:572–5.
Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94:287–94.
Gonzales F, Bruno B, Alarcon Fuentes M, De Berranger E, Guimber D, Behal H, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr. 2017. https://doi.org/10.1016/j.clnu.2017.10.005..
Sheean P, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:656–64.
Kawajiri A, Fuji S, Tanaka J, Kono C, Hirakawa T, Tanaka T, et al. Clinical impact of hyperglycemia on days 0-7 after allogeneic stem cell transplantation. Bone Marrow Transplant. 2017;52:1156–63.
Guieze R, Lemal R, Cabrespine A, Hermet E, Tournilhac O, Combal C, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33:533–8.
Azarnoush S, Bruno B, Beghin L, Guimber D, Nelken B, Yakoub-Agha I, et al. Enteral nutrition: a first option for nutritional support of children following allo-SCT? Bone Marrow Transplant. 2012;47:1191–5.
Bicakli DH, Yilmaz MC, Aksoylar S, Kantar M, Cetingul N, Kansoy S. Enteral nutrition is feasible in pediatric stem cell transplantation patients. Pediatr Blood Cancer. 2012;59:1327–9.
Murray SM, Pindoria S. Nutrition support for bone marrow transplant patients. Cochrane Database Syst Rev. 2009;1:1–74.
Lemal R, Cabrespine A, Pereira B, Combal C, Ravinet A, Hermet E, et al. Could enteral nutrition improve the outcome of patients with haematological malignancies undergoing allogeneic haematopoietic stem cell transplantation? A study protocol for a randomized controlled trial (the NEPHA study). Trials. 2015;16:136.
Flowers ME, McDonald G, Carpenter P, Boeckh M, Sanders J, Deeg J, et al. Long-term follow-up after hematopoietic stem cell transplant; general guideline or referring physicians. Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance, Version 4 August 2008, p. 1–78.
Imataki O, Nakatani S, Hasegawa T, Kondo M, Ichihashi K, Araki M, et al. Nutritional support for patients suffering from intestinal graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81:747–52.
Bassim CW, Fassil H, Dobbin M, Steinberg SM, Baird K, Cole K, et al. Malnutrition in patients with chronic GVHD. Bone Marrow Transplant. 2014;49:1300–6.
Isenring EA, Teleni L. Nutritional counseling and nutritional supplements: a cornerstone of multidisciplinary cancer care for cachectic patients. Curr Opin Support Palliat Care. 2013;7:390–5.
Brown SA, Goringe A, Fegan C, Davies SV, Giddings J, Whittaker JA, et al. Parenteral glutamine protects hepatic function during bone marrow transplantation. Bone Marrow Transplant. 1998;22:281–4.
Goringe AP, Brown S, Callaghan U, Rees J, Jebb S, Elia M, et al. Glutamine and vitamin E in the treatment of hepatic veno-occlusive disease following high-dose chemo- therapy. Bone Marrow Transplant. 1998;22:2879–84.
Wilmore DW, Schloerb PR, Ziegler TR. Glutamine in the support of patients following bone marrow transplantation. Curr Opin Clin Nutr Metab Care. 1999;2:323–7.
Ziegler TR. Glutamine supplementation in cancer patients receiving bone marrow transplantation and high dose chemotherapy. J Nutr. 2001;131:2578S–84S.
da Gama Torres HO, Vilela EG, da Cunha AS, Goulart EM, Souza MH, Aguirre AC, et al. Efficacy of glutamine- supplemented parenteral nutrition on short-term survival following allo-SCT: a randomized study. Bone Marrow Transplant. 2008;41:1021–7.
Noe JE. L-glutamine use in the treatment and prevention of mucositis and cachexia: a naturopathic perspective. Integr Cancer Ther. 2009;8:409–15.
Lye AD, Hayslip JW. Immunonutrition: does it have a role in improving recovery in patients receiving a stem cell transplant? Nutr Cancer. 2012;64:503–7.
Baena-Gomez MA, Aguilar MJ, Mesa MD, Navero JLP, Gil-Campos M. Changes in antioxidant defense system using different lipid emulsions in parenteral nutrition in children after hematopoietic stem cell transplantation. Nutrients. 2015;7:7242–55.
Kota H, Chamberlain RS. Immunonutrition is associated with a decreased incidence of graft-versus-host disease in bone marrow transplant recipients: a meta-analysis. J Parenter Enter Nutr. 2016;41:1286–92.
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–6.
Stebbings S, Munro K, Simon MA, Tannock G, Highton J, Harmsen H, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology. 2002;41:1395–401.
Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 2015;67:686–91.
Hevia A, Milani C, Lopez P, Cuervo A, Arboleya S, Duranti S, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014;5:e01548.
Zhang X, Zhang D, Jia H. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905.
Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–11.
Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–87.
Biagi E, Zama D, Nastasi C, Consolandi C, Fiori J, Rampelli S, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015;50:992–8.
Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:640–5.
Peric Z, Vranjes VR, Durakovic N, Desnica L, Marekovic I, Serventi-Seiwerth R, et al. Gut colonization by multidrug-resistant bacteria is an independent risk factor for development of intestinal acute graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:1221–2.
Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–204.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70.
Sivan A, Corrales L, Hubert N, Willams JB, Acquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9.
Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6.
Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271.
Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–82.
Bilinski J, Robak K, Peric Z, Marchel H, Karakulska-Prystupiuk E, Halaburda K, et al. Impact of gut colonization by antibiotic-resistant bacteria on the outcomes of allogeneic hematopoietic stem cell transplantation: a retrospective, single-center study. Biol Blood Marrow Transplant. 2016;22:1087–93.
Peled J, Devlin S, Staffas A, Lumish M, Khanin R, Littmann E, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35:1650–9.
Gerbitz A, Schultz M, Wilke A, Linde HJ, Schölmerich J, Andreesen R, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103:4365–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.Please confirm the amended 'Conflict of interest' statement.I confirm
Rights and permissions
About this article
Cite this article
Peric, Z., Botti, S., Stringer, J. et al. Variability of nutritional practices in peritransplant period after allogeneic hematopoietic stem cell transplantation: a survey by the Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transplant 53, 1030–1037 (2018). https://doi.org/10.1038/s41409-018-0137-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0137-1
This article is cited by
-
Proactive enteral nutrition for patients undergoing allogeneic stem cell transplantation- implementation and clinical outcomes
European Journal of Clinical Nutrition (2024)
-
The European Society for Blood and Marrow Transplantation (EBMT) roadmap and perspectives to improve nutritional care in patients undergoing hematopoietic stem cell transplantation on behalf of the Cellular Therapy and Immunobiology Working Party (CTIWP) and the Nurses Group (NG) of the EBMT
Bone Marrow Transplantation (2023)
-
Nutrition support and clinical outcomes following allogeneic stem cell transplantation
Bone Marrow Transplantation (2023)
-
Nutrition support use and clinical outcomes in patients with multiple myeloma undergoing autologous stem cell transplant
Supportive Care in Cancer (2022)
-
Mortality and microbial diversity after allogeneic hematopoietic stem cell transplantation: secondary analysis of a randomized nutritional intervention trial
Scientific Reports (2021)