Abstract
Background/ Objectives
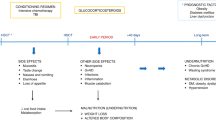
Nutrition support is frequently required post allogeneic haematopoietic progenitor cell transplantation (HPCT) however the tolerance of enteral nutrition (EN) can vary. This mixed methods study aimed to explore staff perceptions, barriers and enablers to the use of EN post HPCT and report the implementation and outcomes of a nutrition protocol.
Subject/ Methods
A survey on barriers and enablers to the use of EN was developed and distributed to medical and nursing staff. Data on nutrition and clinical outcomes was collected for 12 months post implementation of a new nutrition protocol.
Results
Thirty staff completed the survey, key barriers identified included uncertain EN tolerance, lack of confidence in nasogastric tube placement and insufficient training and resources. Eighty-four patients commenced EN, 23 changed to PN (27%) and 61 received EN only (73%). In total 36 patients received PN and eight patients oral nutrition support only. There was a difference in type of conditioning (p = 0.025) and nutritional status (p = 0.016) between patients who received PN vs EN only, with a higher proportion of malnourished patients receiving PN (23% vs 5%). Patients who received PN had a longer length of hospital stay (median 22 vs 19 days, p = 0.012) and lower rate of survival to day 100 (81% vs 95%, p = 0.036) than patients who received EN.
Conclusion
The use of EN may lead to improved clinical outcomes compared to PN therefore should be implemented as first line nutrition support.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets analysed during the current study are not publicly available due to ethics requirements.
References
Dietrich S, Radujkovic A, Stolzel F, Falk CS, Benner A, Schaich M, et al. Pretransplant metabolic distress predicts relapse of acute myeloid leukemia after allogeneic stem cell transplantation. Transplantation. 2015;99:1065–71.
Fuji S, Mori T, Khattry N, Cheng J, Do YR, Yakushijin K, et al. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transpl. 2015;50:100–5.
Peric Z, Botti S, Stringer J, Krawczyk J, van der Werf S, van Biezen A, et al. Variability of nutritional practices in peritransplant period after allogeneic hematopoietic stem cell transplantation: a survey by the complications and quality of life working party of the EBMT. Bone Marrow Transpl. 2018;53:1030–7.
Andersen S, Brown T, Kennedy G, Banks M. Implementation of an evidenced based nutrition support pathway for haematopoietic progenitor cell transplant patients. Clin Nutr. 2015;34:536–40.
Baumgartner A, Bargetzi A, Zueger N, Bargetzi M, Medinger M, Bounoure L, et al. Revisiting nutritional support for allogeneic hematologic stem cell transplantation-a systematic review. Bone Marrow Transpl. 2017;52:506–13.
Andersen S, Weber N, Kennedy G, Brown T, Banks M, Bauer J. Tolerability of proactive enteral nutrition post allogeneic haematopoietic progenitor cell transplant: a randomised comparison to standard care. Clin Nutr. 2020;39:1364–70.
Guieze R, Lemal R, Cabrespine A, Hermet E, Tournilhac O, Combal C, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33:533–8.
Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94:287–94.
Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr. 2019;38:738–44.
Andersen S, Staudacher H, Weber N, Kennedy G, Varelias A, Banks M, et al. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br J Haematol. 2020;188:570–81.
Andersen S, Kennedy G, Banks M. A randomised controlled comparison of enteral versus parenteral nutritional support post allogeneic haematopoietic cell transplantation. Clin Nutr ESPEN. 2015;10:e102–e6.
Seguy D, Berthon C, Micol JB, Darre S, Dalle JH, Neuville S, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006;82:835–9.
Andersen S, Banks M, Brown T, Weber N, Kennedy G, Bauer J. Nutrition support during allogeneic stem cell transplantation: evidence versus practice. Support Care Cancer. 2020;28:5441–7.
Cohen J, Wakefield CE, Tapsell LC, Walton K, Cohn RJ. Parent, patient and health professional perspectives regarding enteral nutrition in paediatric oncology. Nutr Diet. 2017;74:476–87.
Montgomery K, Belongia M, Schulta C, Mulberry MH, Nugent ML, Simpson PM. Health care providers’ perceptions of nutrition support in pediatric oncology and hematopoietic stem cell transplant patients. J Pediatr Oncol Nurs. 2016;33:265–72.
Williams-Hooker R, Adams M, Havrilla DA, Leung W, Roach RR, Mosby TT. Caregiver and health care provider preferences of nutritional support in a hematopoietic stem cell transplant unit. Pediatr Blood Cancer. 2015;62:1473–6.
Cahill NE, Jiang X, Heyland DK. Revised questionnaire to assess barriers to adequate nutrition in the critically Ill. JPEN J Parenter Enter Nutr. 2014;40:511–8.
Cahill NE, Murch L, Cook D, Heyland DK. Barriers to feeding critically ill patients: a multicenter survey of critical care nurses. J Crit Care. 2012;27:727–34.
Cahill NE, Murch L, Wang M, Day AG, Cook D, Heyland DK. The validation of a questionnaire to assess barriers to enteral feeding in critically ill patients: a multicenter international survey. BMC Health Serv Res. 2014;14:197.
Leong AY, Cartwright KR, Guerra GG, Joffe AR, Mazurak VC, Larsen BM. A Canadian survey of perceived barriers to initiation and continuation of enteral feeding in PICUs. Pediatr Crit Care Med. 2014;15:e49–55.
Van Limbergen J, Haskett J, Griffiths AM, Critch J, Huynh H, Ahmed N, et al. Toward enteral nutrition for the treatment of pediatric Crohn disease in Canada: a workshop to identify barriers and enablers. Can J Gastroenterol Hepatol. 2015;29:351–6.
Finch TL, Girling, M, May, CR, Mair, FS, Murray, E, Treweek, S, et al. NoMAD: implementation measure based on normalization process theory. Measurement instrument. 2015.
Cahill NE, Day AG, Cook D, Heyland DK. Development and psychometric properties of a questionnaire to assess barriers to feeding critically ill patients. Implement Sci. 2013;8:140.
Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. 2016;11:33.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Thomas ED, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Bone-marrow transplantation. N. Engl J Med. 1975;292:895–902.
World Health Organisation. WHO Handbook for reporting results of cancer treatment WHO Offset Publication (1979).
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Huang J, Yang L, Zhuang Y, Qi H, Chen X, Lv K. Current status and influencing factors of barriers to enteral feeding of critically ill patients: a multicenter study. J Clin Nurs. 2019;28:677–85.
Habschmidt MG, Bacon CA, Gregoire MB, Rasmussen HE. Medical nutrition therapy provided to adult hematopoietic stem cell transplantation patients. Nutr Clin Pract. 2012;27:655–60.
Langdana A, Tully N, Molloy E, Bourke B, O’Meara A. Intensive enteral nutrition support in paediatric bone marrow transplantation. Bone Marrow Transpl. 2001;27:741–6.
Doney K, McMillen K, Buono L, Deeg HJ, Gooley T. Impact of body mass index on outcomes of hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transpl. 2019;25:613–20.
Hirose EY, de Molla VC, Gonçalves MV, Pereira AD, Szor RS, da Fonseca ARBM, et al. The impact of pretransplant malnutrition on allogeneic hematopoietic stem cell transplantation outcomes. Clin Nutr ESPEN. 2019;33:213–19.
Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy K, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117.
Funding
This research was supported by grants from the Royal Brisbane & Women’s Hospital Research Foundation. The funding body has no role in the study design, data collection, analysis, interpretation or in the manuscript preparation.
Author information
Authors and Affiliations
Contributions
Phase one study: SA: conceptualisation, methodology, funding acquisition, investigation, formal analysis, writing original draught, writing review and editing, project administration. NW: methodology, writing review and editing. TB: methodology, writing review and editing. GK: methodology, writing review and editing. MB: conceptualisation, methodology, writing review and editing. JB: conceptualisation, methodology, writing review and editing. Phase two study: SA: conceptualisation, methodology, investigation, formal analysis, writing original draught, writing review and editing. RF: conceptualisation, methodology, investigation, writing review and editing. TB: methodology, writing review and editing. GK: methodology, writing review and editing. MB: methodology, writing review and editing. JB: writing review and editing. DW: methodology, writing review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Phase one study: Approved by the RBWH Human Research Ethics Committee (HREC/18/QRBW/321) and University of Queensland Human Research Ethics Board (approval 2018001520). Phase two study: received an exemption from ethics approval from the RBWH Human Research Ethics Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Andersen, S., Fichera, R., Banks, M. et al. Proactive enteral nutrition for patients undergoing allogeneic stem cell transplantation- implementation and clinical outcomes. Eur J Clin Nutr 78, 251–256 (2024). https://doi.org/10.1038/s41430-023-01367-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01367-8