Abstract
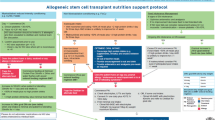
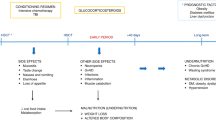
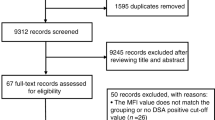
Nutrition support is frequently required post allogeneic stem cell transplantation (SCT) and while there is some evidence on the benefits of enteral nutrition (EN), parenteral nutrition (PN) is widely used in practice. The study aimed to examine the impact of EN versus PN on early outcomes following SCT. All patients who underwent allogeneic SCT over 2.5 years were included in the analysis. Data was retrospectively collected on mode of nutrition support with clinical outcome data obtained from an existing database. Clinical outcomes were compared between groups by logistic, poisson and negative binomial regression, with adjustment for baseline confounders as appropriate. Patients who received EN then changed to PN had a longer length of hospital stay compared to those who received EN only (IR 1.24, 95% CI: 1.11–1.38, p < 0.001). Compared to those who received EN only, patients who received EN that changed to PN or PN only had a longer time to neutrophil engraftment (IR 1.11, 95% CI: 1.02–1.20, p = 0.016 and IR 1.16, 95% CI: 1.03–1.30, p = 0.017) and platelet engraftment (IR 1.20, 95% CI 1.08–1.33, p < 0.001 and IR 1.24, 95% CI 1.08–1.42, p = 0.002). Enteral nutrition should be first line nutritional support for patients undergoing allogeneic SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Baumgartner A, Zueger N, Bargetzi A, Medinger M, Passweg JR, Stanga Z, et al. Association of Nutritional Parameters with Clinical Outcomes in Patients with Acute Myeloid Leukemia Undergoing Haematopoietic Stem Cell Transplantation. Ann Nutr Metab. 2016;69:89–98.
El-Ghammaz A, Ben Matoug R, Elzimaity M, Mostafa N, El-Ghammaz AMS. Nutritional status of allogeneic hematopoietic stem cell transplantation recipients: influencing risk factors and impact on survival. Support Care Cancer. 2017;25:3085–93.
Fuji S, Mori T, Khattry N, Cheng J, Do YR, Yakushijin K, et al. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transpl. 2015;50:100–5.
Andersen S, Weber N, Kennedy G, Brown T, Banks M, Bauer J. Tolerability of proactive enteral nutrition post allogeneic haematopoietic progenitor cell transplant: A randomised comparison to standard care. Clin Nutr. 2020;39:1364–70.
Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation 2012;94:287–94.
Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr. 2019;38:738–44.
Guieze R, Lemal R, Cabrespine A, Hermet E, Tournilhac O, Combal C, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33:533–8.
Andersen S, Staudacher H, Weber N, Kennedy G, Varelias A, Banks M, et al. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br J Haematol. 2020;188:570–81.
Skaarud KJ, Hjermstad MJ, Bye A, Veierod MB, Gudmundstuen AM, Lundin KEA, et al. Effects of individualized nutrition after allogeneic hematopoietic stem cell transplantation following myeloablative conditioning; a randomized controlled trial. Clin Nutr Espen. 2018;28:59–66.
August DA, Huhmann MB, A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. J Parenter Enter Nutr. 2009;33:472–500.
Barban JB, Simoes BP, Moraes B, Anunciacao CRD, Rocha CSD, Pintor DCQ, et al. Brazilian Nutritional Consensus in Hematopoietic Stem Cell Transplantation: Adults. Einstein (Sao Paulo, Braz). 2020;18:Ae4530.
Andersen S, Banks M, Brown T, Weber N, Kennedy G, Bauer J. Nutrition support during allogeneic stem cell transplantation: evidence versus practice. Support Care Cancer. 2020;28:5441–7.
Peric Z, Botti S, Stringer J, Krawczyk J, van der Werf S, van Biezen A, et al. Variability of nutritional practices in peritransplant period after allogeneic hematopoietic stem cell transplantation: a survey by the Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transpl. 2018;53:1030–7.
Baumgartner A, Bargetzi M, Bargetzi A, Zueger N, Medinger M, Passweg J, et al. Nutritional support practices in hematopoietic stem cell transplantation centers: A nationwide comparison. Nutrition 2017;35:43–50.
Thomas EDSR, Clift RA, Fefer A, Johnson FL, Neiman PE, Lerner KG, et al. Bone-marrow transplantation. N. Engl J Med. 1975;292:895–902.
World Health Organisation. WHO Handbook for reporting results of cancer treatment WHO Offset Publication 1979.
Kolbe K, Domkin D, Derigs HG, Bhakdi S, Huber C, Aulitzky WE. Infectious complications during neutropenia subsequent to peripheral blood stem cell transplantation. Bone Marrow Transpl. 1997;19:143–7.
Szeluga DJ, Stuart RK, Brookmeyer R. Nutritional support of bone marrow transplant recipients: A prospective, randomized clinical trial comparing total parenteral nutrition to an enteral feeding program. Cancer Res. 1987;47:3309–16.
Andersen S, Kennedy G, Banks M. A randomised controlled comparison of enteral versus parenteral nutritional support post allogeneic haematopoietic cell transplantation. Clin Nutr ESPEN. 2015;10:e102–e6.
Zama D, Gori D, Muratore E, Leardini D, Rallo F, Turroni S, et al. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: a Systematic Review and Meta-Analysis. Transpl Cell Ther. 2021;27:180.e1–e8.
Mancini N, Greco R, Pasciuta R, Barbanti MC, Pini G, Morrow OB, et al. Enteric Microbiome Markers as Early Predictors of Clinical Outcome in Allogeneic Hematopoietic Stem Cell Transplant: Results of a Prospective Study in Adult Patients. Open Forum Infectious Dis. 2017;4.
Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharm Ther. 2015;42:515–28.
Romick-Rosendale LE, Haslam DB, Lane A, Denson L, Lake K, Wilkey A, et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transpl. 2018;24:2418–24.
Ralls MDF, Feng Y, Ignatoski K, Teitelbaum D. Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function. Surgery 2015;157:732–42.
Fegan C, Poynton CH, Whittaker JA. The gut mucosal barrier in bone marrow transplantation. Bone Marrow Transpl. 1990;5:373.
Johansson JE, Ekman T. Gastro-intestinal toxicity related to bone marrow transplantation: disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transpl. 1997;19:921–5.
Balian C, Garcia M, Ward J. A Retrospective Analysis of Bloodstream Infections in Pediatric Allogeneic Stem Cell Transplant Recipients: The Role of Central Venous Catheters and Mucosal Barrier Injury. J Pediatr Oncol Nurs. 2018;35:210–7.
Hu LJ, Wang Q, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Positive stool culture could predict the clinical outcomes of haploidentical hematopoietic stem cell transplantation. Front Med. 2019;13:492–503.
Lough M, Watkins R, Campbell M, Carr K, Burnett A, Shenkin A. Parenteral nutrition in bone marrow transplantation. Clin Nutr. 1990;9:97–101.
Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transpl. 2006;38:629–33.
Skop-Lewandowska A, Kolarzyk E, Skotnicki AB. Importance of parenteral nutrition in patients undergoing allogeneic hematopoietic stem cell transplantation. Onkologie 2011;34:210–2.
Author information
Authors and Affiliations
Contributions
SA completed the literature review, protocol writing, ethics submissions and manuscript drafting, JX completed the data collection and manuscript drafting, SL completed the data analysis and all authors contributed to study design, manuscript review and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andersen, S., Xu, J., Llewellyn, S. et al. Nutrition support and clinical outcomes following allogeneic stem cell transplantation. Bone Marrow Transplant 58, 1137–1142 (2023). https://doi.org/10.1038/s41409-023-02080-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02080-7