Abstract
Study design
Single-center, retrospective case-control study.
Objectives
This study aimed to determine the risk factors for progression of neurological symptoms after anterior fusion for cervical spine trauma with no or incomplete spinal cord injury.
Setting
Community-based hospital with an acute care center in Japan.
Methods
We retrospectively reviewed 54 consecutive unstable subaxial cervical spine fracture/dislocation cases that had undergone surgical treatment. A total of 20 patients with no or incomplete spinal cord injury who underwent anterior fusion were identified. Injury characteristics, bony spinal canal diameter (SCD) at the injured level on computed tomography (CT), diagnosis delay of more than 24 h, and other surgery-related parameters were documented as potential risk factors.
Results
The study population included 16 male and 4 female patients. The median age was 71.5 (range: 20–88) years. Two cases of SCI progression were identified (AIS E to C5-8 C and AIS D to C5-8 C). Both cases occurred in men who were older than the average age of all the patients. Only delayed diagnosis was significantly associated with the progression of SCI (p = 0.02). SCDs on CT demonstrated a tendency to be smaller than those of cases without progression, but this was not statistically significant (progression: median, 8.1 [7.2–8.9] mm; no progression: median, 10.1 [4.2–12.6] mm; p = 0.21).
Conclusion
Our results suggested that a delay in diagnosis was associated with neurological progression after ACF. Furthermore, imposing ligamentous flavum might become a compression factor if the diagnosis is delayed.
Similar content being viewed by others
Introduction
Anterior cervical fusion (ACF) is a common procedure for the treatment of various cervical spinal conditions. ACF is often used for unstable cervical spine trauma associated with spinal cord injury (SCI) and makes it possible to directly remove anterior compressive factors, such as herniated discs and bone fragments, which cannot be approached from the posterior side [1]. Another potential benefit of ACF for acutely traumatized patients is that it is performed in the supine position, without subjecting the patient to significant position changes, thus reducing the risk of unacceptable excessive motion in injured segments [2] and systemic hemodynamic changes [3]. There are certain situations for which the posterior approach is preferable [4], such as unreduced facet dislocations, end-plate compression fractures associated with facet fracture [5], ankylosing spine [6], and severe osteoporosis. For other cases, either approach can be applied based on the surgeons’ preferences and ACF has demonstrated clinical results similar to posterior procedures [7, 8] and was reported to be associated with shorter operative times and less blood loss [9]. Neurological complications are rare among non-trauma patients undergoing ACF, with a reported incidence of 0–1.6% of all ACF patients [10, 11]. However, the incidence of neurological complications of cervical spine trauma patients can be higher, with reports of up to 6.0% of ACF cases [12,13,14,15,16]. The definition of a neurological complication differs from study to study, and it may comprise not only SCI but also nerve root symptoms and even recurrent nerve injury. Although SCI is one of the most catastrophic postoperative sequelae associated with ACF, it remains unclear what factors are associated with new-onset SCI or progression of incomplete SCI. The purpose of this pilot study was to explore the risk factors for the progression or onset of SCI after ACF performed for trauma.
Methods
Institutional review board approval was obtained for this study. We retrospectively reviewed 54 consecutive unstable subaxial cervical spine fracture/dislocation cases that underwent surgical treatment between 2004 and 2017 at a single community-based tertiary institute. A total of 17 cases were treated with posterior procedures only, 4 cases were treated with a combined approach, and 33 cases were treated with ACF. Eight patients with complete palsy (American Spinal Injury Association [ASIA] Impairment Scale [AIS] grade A [17]) and two patients with severe head trauma were excluded because of difficulty in assessing a detailed course of neurological symptoms. Among the 23 remaining cases, 20 had complete data that were included in the analysis. The progression of SCI was defined as a new postoperative neurological deficit consistent with SCI in patients without preoperative neurological deficits, more than one grade of progression according to the AIS, or more than one level of upward progression of SCI in patients with preoperative incomplete SCI. Neurological symptoms caused by nerve root injury or recurrent nerve injury were excluded from neurological progression.
Injury level, preoperative AIS grade (B–E) [17], Allen classification [18], preoperative focal alignment (kyphotic, neutral, and lordotic) determined by the focal angular change of the estimated curvature line on the initial lateral radiograph of the cervical spine for patients without unilateral/bilateral complete facet dislocation or on the postreduction lateral radiograph if the patient had a complete dislocation, the bony spinal canal diameter (SCD) at the injured level on computed tomography (CT), diagnosis delayed more than 24 h (considered as a missed diagnosis at the initial medical examination or delayed presentation), time between injury and surgery, operation time, intraoperative accidental durotomy, and other preoperative/intraoperative events that might be related to progression of SCI were documented as potential risk factors. Causes of deterioration were determined by a board-certified orthopedic spine surgeon with no affiliation with the direct care of these patients by reviewing operative records of the initial surgery, pre-deterioration and post-deterioration imaging studies (radiograph, CT, and magnetic resonance imaging [MRI]), as well as operative records of the salvage procedures.
The treatment protocol for subaxial cervical spine trauma during the study period was as follows: (1) immediate closed reduction was conducted if the patient had an unreduced facet dislocation at the initial encounter; (2) if the closed reduction failed or if the patient had neurological deficits with obvious spinal cord compression, then surgical treatment was urgently warranted; and (3) if the patients did not have either neurological deficits or obvious spinal cord compression on imaging studies, then temporary immobilization with a hard collar or halo vest along with bed rest and adequate precautions during nursing care were performed. Definitive fixation was performed at the earliest available opportunity as a planned surgery.
The ACF surgical procedure was standard discectomy/partial corpectomy with an iliac crest tri-cortical autologous graft and anterior plate instrumentation [19]. Partial corpectomy was performed in all cases. The thin posterior part of the vertebral body was preserved to obtain the maximum contact area with the graft. Fragments under the posterior bony wall were carefully inspected by utilizing the removed disc space. The posterior longitudinal ligament (PLL) was usually preserved to maintain stability. Complete removal of the vertebral posterior wall and/or resection of PLL were only performed for patients with obvious anterior cord compressing factors according to preoperative imaging studies. After interpreting postoperative imaging studies, it was determined that no patient in this study population underwent complete posterior vertebral wall resection. All surgeries were performed by or under the direct supervision of board-certified orthopedic surgeons who had more than 10 years of experience with spinal trauma care.
A comparison between patients with and without progression was performed using the Mann–Whitney U test for continuous and ordinal variables and the Fisher exact test for categorical variables. Two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed using EZR (version 1.3.7) [20], which is a free statistical software based on the R software environment (R for 3.1.0 GUI 1.64).
Results
The study population consisted of 16 male and 4 female patients. The median age was 71.5 (range: 20–88) years. Causes of injury were traffic accident (n = 4), falling from a height (n = 7), toppling over (n = 8), and others (n = 1). No patient was treated with posterior decompression at the time of the first surgery. There was no case of preoperative deterioration during the study period.
Two cases of SCI progression were identified (AIS E to C [C6] and AIS D [C6] to C [C6]). Both cases were observed in male patients who were older than the average age of all patients. Only delayed diagnosis was significantly associated with SCI progression (p = 0.02). SCDs on CT were smaller than those of cases without progression, but this was not statistically significant (progression: median, 8.1 (7.2–8.9) mm; no progression: median, 10.1 (4.2–12.6) mm; p = 0.21). Although there was no statistical significance between the two groups, notably, the time from injury to surgery was substantially long for both groups during the study period (progression: median, 15.5 [3–28] days; no progression: median, 8.8 (0.8–43) days; p = 0.89). This could be explained by the historical trend for the timing of surgery during the early period, the number of elderly patients with medical comorbidities, and the number of patients with delayed referral. Significant differences and tendencies were not observed for other variables. A summary of the results is shown in Tables 1 and 2.
Preoperative and intraoperative hypotensive/hypoxic events were not recorded. No incidental durotomy or other intraoperative technical issues that might lead to SCI were documented.
The details of deteriorated cases are described in Table 3. Both patients had anterior subluxation at the time of the correct diagnosis. The displacement was easily reduced without neurological deterioration with neck extension, but it easily recurred with neck flexion for both. One patient (Fig. 1) demonstrated no postoperative neurological deficits on the day of surgery, but muscle weakness in all extremities was observed the next morning. The other patient (Fig. 2) reported slight weakness in the right upper extremity; however, no other changes occurred immediately after surgery. The palsy progressed to the bilateral lower legs during the hours after surgery.
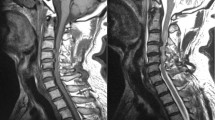
Imaging studies of a 71-year-old man with deterioration (case 1). a Computed tomography (CT) on the day of admission showing C5/6 subluxation and focal kyphotic deformity (arrow). b Preoperative magnetic resonance imaging (MRI) showing anterior subluxation of C5/6 and spinal canal stenosis due to traumatic herniation of the C5/6 disc (arrow). c Postoperative MRI showing spinal cord compression due to the remaining anterior herniated disc (arrow) and imposing ligamentous flavum (arrowhead)
Imaging studies of an 82-year-old man with deterioration (case 2). a Magnetic resonance imaging (MRI) during the initial hospital visit showing high-intensity lesions in the spinal cord at the C6-7 level (arrow) with no evidence of fracture or dislocation. b Dynamic radiographic imaging on day 21 of follow-up showing anterior dislocation at the C6/C7 level (arrow). c Postoperative MRI showing worsening of canal stenosis at the C6/C7 level and adjacent C5/C6 level (arrowhead) and a low-intensity structure at the C6/C7 level posteriorly (arrow). This structure was confirmed as an imposed ligamentous flavum
Discussion
In this pilot study, we demonstrated that a delayed diagnosis can be a statistically significant risk factor for neurological progression after ACF among trauma patients. A delayed diagnosis is known to be associated with the incidence of preventable neurological injuries among spinal trauma patients [21]; however, there are no reports regarding the incidence of postoperative neurological complications. Both cases of progression demonstrated spinal cord compression due to imposing ligamentous flavum. Rhee et al. reported two cases of postoperative neurological deterioration due to an imposing ligamentous flavum [22]. One case considerably resembled one of our cases. A 66-year-old patient who was diagnosed 6 days after his injury and underwent circumferential fusion without posterior decompression demonstrated SCI progression after surgery. The delayed diagnosis might have been associated with flavum hypertrophy. One possible reason for this was that prolonged kyphotic alignment of the subluxated cervical spine might have been complicated with an adhesion between the ruptured flavum and dural sac; therefore, it would have been prone to unusual positioning of the flavum, causing spinal cord compression after surgical correction. Although the fracture pattern of the Allen classification and preoperative alignment did not show statistical significance in our study, all our cases and Rhee’s reported cases involved anterior dislocation injuries associated with focal kyphotic alignment.
SCI progression can occur in patients with posterior fixation. Sugimoto reported two cases of postoperative deterioration after posterior fusion for fractures of ankylosed spines [23]. The patients had spinal canal stenosis and were treated without decompression. It is still unclear whether the likelihood of neurological progression due to this mechanism differed according to the surgical approach. More meticulous bone excision and resection of the PLL might have prevented neurological deterioration in these cases. According to the operative records, disruption of the interspinous ligament at the index level and slight movement of the laminae, even after ACF, were observed and imposed flavum was confirmed through the interlaminal space. When the posterior approach is chosen for the primary surgery, the imposing flavum might be noticed during the procedure.
In our cohort, the time from injury to surgery was not significantly associated with postoperative neurological progression. This may imply that temporal stabilization in the anatomical alignment and adequate care for the unstable spine to minimize excessive movement is essential for preventing not only preoperative but also postoperative neurological progression.
Although the SCD was not statistically significant, those of cases with progression were smaller than those of cases without progression in our cohort. Relatively small spinal canals were also noted in a previous report [22]. Under conditions of cervical spinal canal stenosis, a small amount of additional compression that does not cause any symptoms in patients with normal SCD can become an important issue. Degenerative spinal canal stenosis is a known risk factor for SCI among elderly people after minor trauma [24]. Aebli et al. reported that an SCD less than 8 mm on MRI was the best cutoff point for predicting whether minor trauma caused SCI in elderly people [25]. Our patients with neurological progression had SCDs of 7.2 and 8.9 mm on CT. Because the SCD on CT myelography was reported to be approximately 10% larger than that observed with MRI measurements [26], and because the bony SCD on CT was larger than the SCD on CT myelography, the SCDs on MRI of our patients with progression were highly likely to be estimated as smaller than 8.0 mm. We believe that the cutoff point of Aebli et al. can also be applied to identify groups at high risk for postsurgical neurological progression.
As this study was intended to identify candidate variables as potential risk factors for future studies, the study had several inherent limitations. The first and most substantial limitation was the small sample size. Second, this was a single-center retrospective study. Third, our analyses did not include information regarding concomitant injuries or medical comorbidities. Finally, one can argue that the incidence of neurological progression in this study was unacceptably higher than that of other studies. We think this is accounted for by the higher proportion of patients with older ages and the prevalence of cervical spinal canal stenosis in our cohort. The percentages of patients older than 60 years and 70 years in our cohort were approximately 70% and 50%, respectively; to the best of our knowledge, our cohort possessed the most aged individuals compared with similar studies [7,8,9, 12,13,14,15, 27]. To overcome these limitations and reach an agreeable conclusion, a multicenter study with a larger case volume and data including comorbidities, spinal canal status, and surgical details is warranted to identify risk factors for neurological deterioration among patients with cervical spine trauma.
In summary, we demonstrated that a delay in diagnosis was associated with neurological progression after ACF, and that imposing ligamentous flavum might become a compression factor if the diagnosis is delayed. Furthermore, SCD was smaller among patients with progression. These factors should be included in future studies of neurological complications of cervical spine trauma surgery. In cases of cervical spine injury with these factors, additional posterior decompression or more meticulous bone excision during anterior fusion might be beneficial preventive measures for clinical implementation.
References
Neves N. Compression (AO type-A injuries). In: Vialle LR, Oner FC, Vaccaro AR, editors. AOSpine masters series, volume 5: cervical spine trauma. New York: Thieme; 2015. p. 83–93.
Conrad BP, Horodyski M, Wright J, Ruetz P, Rechtine GR. Log-rolling technique producing unacceptable motion during body position changes in patients with traumatic spinal cord injury. J Neurosurg Spine. 2007;6:540–3.
Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. 2008;100:165–83.
Dvorak MF, Fisher CG, Fehlings MG, Rampersaud YR, Öner FC, Aarabi B, et al. The surgical approach to subaxial cervical spine injuries: an evidence-based algorithm based on the SLIC classification system. Spine. 2007;32:2620–9.
Johnson MG, Fisher CG, Boyd M, Pitzen T, Oxland TR, Dvorak MF. The radiographic failure of single segment anterior cervical plate fixation in traumatic cervical flexion distraction injuries. Spine. 2004;29:2815–20.
Einsiedel T, Schmelz A, Arand M, Wilke HJ, Gebhard F, Hartwig E, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine. 2006;5:33–45.
Lambiris E, Kasimatis GB, Tyllianakis M, Zouboulis P, Panagiotopoulos E. Treatment of unstable lower cervical spine injuries by anterior instrumented fusion alone. J Spinal Disord Tech. 2008;21:500–7.
Brodke DS, Anderson PA, Newell DW, Grady MS, Chapman JR. Comparison of anterior and posterior approaches in cervical spinal cord injuries. J Spinal Disord Tech. 2003;16:229–35.
Belirgen M, Dlouhy BJ, Grossbach AJ, Torner JC, Hitchon PW. Surgical options in the treatment of subaxial cervical fractures: a retrospective cohort study. Clin Neurol Neurosurg. 2013;115:1420–8.
Arshi A, Wang C, Park HY, Blumstein GW, Buser Z, Wang JC. et al. Ambulatory anterior cervical discectomy and fusion is associated with a higher risk of revision surgery and perioperative complications: an analysis of alarge nationwide database. Spine J. 2018;18:1180–87.
Tasiou A, Giannis T, Brotis AG, Siasios I, Georgiadis I, Gatos H, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg. 2017;3:444–59.
Kasimatis GB, Panagiotopoulos E, Gliatis J, Tyllianakis M, Zouboulis P, Lambiris E. Complications of anterior surgery in cervical spine trauma: an overview. Clin Neurol Neurosurg. 2009;111:18–27.
Anissipour AK, Agel J, Baron M, Magnusson E, Bellabarba C, Bransford RJ. Traumatic cervical unilateral and bilateral facet dislocations treated with anterior cervical discectomy and fusion has a low failure rate. Global Spine J. 2017;7:110–5.
Razack N, Green BA, Levi AD. The management of traumatic cervical bilateral facet fracture-dislocations with unicortical anterior plates. J Spinal Disord. 2000;13:374–81.
Marshall LF, Knowlton S, Garfin SR, Klauber MR, Eisenberg HM, Kopaniky D, et al. Deterioration following spinal cord injury. J Neurosurg. 1987;66:400–4.
Harrop JS, Sharan AD, Vaccaro AR, Przybylski GJ. The cause of neurologic deterioration after acute cervical spinal cord injury. Spine. 2001;26:340–6.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med. 2011;34:535–46.
Allen BL Jr., Ferguson RL, Lehmann TR, O’Brien RP. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7:1–27.
Anderson D, Hilibrand A. Procedures 6: anterior cervical corpectomy/diskectomy. In: Baron E, Vaccaro A, editors. Operative techniques: spine surgery. 1st ed. Philadelphia: Saunders Elsevier; 2007. p. 65–74.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Levi AD, Hurlbert RJ, Anderson P, Fehlings M, Rampersaud R, Massicotte EM, et al. Neurologic deterioration secondary to unrecognized spinal instability following trauma—a multicenter study. Spine. 2006;31:451–8.
Rhee JM, Kimmerly WS, Smucker JD. Infolding of the ligamentum flavum: a cause of spinal cord compression after reduction of cervical facet injuries. J Spinal Disord Tech. 2006;19:208–12.
Sugimoto Y, Ito Y, Tanaka M, Tomioka M, Hasegawa Y, Nakago K, et al. Cervical cord injury in patients with ankylosed spines: progressive paraplegia in two patients after posterior fusion without decompression. Spine. 2009;34:E861–3.
Regenbogen VS, Rogers LF, Atlas SW, Kim KS. Cervical spinal cord injuries in patients with cervical spondylosis. Am J Roentgenol. 1986;146:277–84.
Aebli N, Ruegg TB, Wicki AG, Petrou N, Krebs J. Predicting the risk and severity of acute spinal cord injury after a minor trauma to the cervical spine. Spine J. 2013;13:597–604.
Grams AE, Gempt J, Förschler A. Comparison of spinal anatomy between 3-Tesla MRI and CT-myelography under healthy and pathological conditions. Surg Radiol Anat. 2010;32:581–5.
Sapkas GS, Papadakis SA. Neurological outcome following early versus delayed lower cervical spine surgery. J Orthop Surg (Hong Kong). 2007;15:183–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Okano, I., Midorikawa, Y., Midorikawa, N. et al. Risk factors for spinal cord injury progression after anterior fusion for cervical spine trauma: a retrospective case-control study. Spinal Cord Ser Cases 4, 90 (2018). https://doi.org/10.1038/s41394-018-0123-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-018-0123-2