Abstract
Cannabidiol (CBD) is widely used and believed to be non-intoxicating, lacking acute performance effects (e.g., non-impairing). However, a synthesis of data has not evaluated this. This meta-analysis synthesized data from controlled human laboratory studies that evaluated if acute CBD use impairs performance. Performance on objective and subjective measures of cognitive and psychomotor function were used as markers for potential performance changes and impairment. Studies were identified through systematic database searches. Adult clinical trials measuring acute CBD effects (within 0–8 h of administration) were included. The primary outcome was the peak mean difference in performance measures between CBD and placebo. A secondary analysis utilizing delta-9-tetrahydrocannabinol (Δ9-THC) as a positive control for comparison to CBD was completed. Pooled Hedges’ g estimates were calculated using robust variance estimation (RVE) meta-regression. The omnibus RVE meta-analysis indicated a statistically significant, but small effect size (Hedge’s g < 0.2) for impaired performance following acute CBD consumption compared to placebo (N = 16 trials, Hedges’ g = 0.122, 95% CI: 0.023–0.221, p = 0.019). Measure type was a significant moderator with larger mean differences between CBD and placebo when subjective measures, specifically self-reported sedation, were used versus objective performance tasks (Hedges’ gSubjective = 0.288 versus Hedges’ gObjective = 0.048). Δ9-THC had a significantly greater magnitude of impairment compared to CBD (N = 8, Hedges’ g = 0.416, 95% CI: 0.017–0.816, p = 0.043). In summary, acute CBD consumption was associated with a small increase in subjective ratings of sedation, but no difference from placebo was observed across multiple domains of objectively assessed cognitive or psychomotor performance. These findings suggest that acute CBD alone is unlikely to significantly impair daily functioning or workplace performance.
Similar content being viewed by others
Introduction
The global increase in legal access to cannabis and cannabinoid-based products for medical and non-medical purposes has been paralleled by their widespread promotion and use. In particular, North America has the highest rate of cannabis use in the world, where some of the first jurisdictions to legalize cannabis exist [1,2,3]. In recent years, North America has been reported to have the highest prevalence of past-year cannabis use compared to other sub-regions globally at 14.5% [4]. Of the emerging cannabinoids available, cannabidiol (CBD), a principal cannabinoid presumed to be non-euphoric and non-intoxicating, has the highest prevalence of past-year use [5]. Past-year CBD use was reported to be 26.1% in the United States and 16.2% in Canada [6]. Common reported reasons for use include medical indications such as the management of pain, anxiety, and depression [6].
This surge in consumption and access to cannabis and cannabinoid products has sparked concerns regarding their careful use during ‘safety-sensitive’ work or activities (e.g., operating motor vehicles or machinery) [7, 8]. To date, a majority of research and public policy has focused on identifying and mitigating cannabis impairment risk related to delta-9-tetrahydrocannabinol (Δ9-THC), the main psychoactive cannabinoid known to produce acute deficits in cognitive performance and driving ability [9, 10]. In contrast, little attention has been given to CBD due to the general belief that it is non-impairing. Although the available evidence has pointed to a lack of cognitive, psychomotor, or subjective effects with oral and vapourized CBD even at high or supratherapeutic doses [11,12,13], there has yet to be a comprehensive, systematic review of the literature to synthesize data on the performance effects of acute CBD exposure, or evaluation of potential moderating factors that may impact sensitivity to performance effects.
This lack of clarity surrounding the effects of CBD on daily functioning presents several concerns. A primary concern is the potential public health consequences for traffic safety if people using CBD are operating motor vehicles under the assumption that it is non-impairing. It is equally as important to consider the implications of CBD-related impairment on workplace health, safety, and policy. At present, many workplaces err on the side of caution and treat cannabis as a single entity, subjecting CBD to the same restrictions as Δ9-THC [14, 15]. These restrictions are particularly relevant to people who use CBD for medical purposes to help manage symptoms or a condition, such as chronic pain, epilepsy, or anxiety when Δ9-THC cannot be used safely [16, 17]. In this context, CBD use may afford individuals the ability to engage in daily activities and workplace duties which they may otherwise be unable to do. Hence, efforts to clarify the risk of CBD-associated impairment are greatly needed to inform public health legislation, as well as workplace policy and practice.
The current meta-analysis synthesized and critically evaluated available evidence from human laboratory studies assessing the potential for CBD to impair cognitive and psychomotor performance. These effects were compared to a placebo control group and a positive control of Δ9-THC. Moderators were also evaluated to determine individual difference and product-specific factors that may alter the magnitude of effect.
Methods
Search strategy
This review was registered on PROSPERO (The International Prospective Register of Systematic reviews) (CRD42021247522) and reported in accordance with PRISMA guidelines (eTable 1) [18]. A systematic search in AMED, EMBASE, CENTRAL, PsychINFO, CINAHL, Clinicaltrials.gov, Medline, MedRxiv, and Web of Science was completed on June 22nd, 2022 and updated again on January 4th, 2023. Literature searches using the keywords associated with cannabidiol or CBD paired with cogniti*, or impair*, or domain-specific keywords (e.g., memory) were independently conducted by two reviewers. As an example, studies were identified by the following expression: (cogniti* OR driving OR coordinat* OR processing speed OR reaction time OR executive function OR memory OR “task performance and analysis” OR attention OR learn* OR task switching OR intoxic* OR motor OR impair* OR perform*) AND (cannabidiol* OR CBD OR Epidiolex OR Epidyolex). The star symbol (*) was used to capture derivatives of search terms (by suffixation) and enclosed quotation marks were used to capture exact phrases. See eTable 2 for full search strategies.
Eligibility criteria and study selection
For inclusion, studies had to meet the following criteria: (1) involve adult participants; (2) placebo-controlled experimental design; (3) report route of cannabinoid administration and dose schedule; (4) measures of self-report, researcher observation, or objective neurocognitive or psychomotor assessments within 0–8 h of CBD administration. CBD administration was defined as administration of any form of CBD either in isolation or with a THC content of <1%. Self-reported/subjective measures of neurocognitive performance were restricted to those with specific constructs (e.g., alert, sedation). Subjective ratings of drug high or intoxication were excluded due to lack of specificity. Only full-length, English-language original research articles were accepted. Studies were excluded if: (1) performance test(s) were not administered within 8 h of CBD administration; (2) either the dose of CBD administered, or the length of time between CBD administration and the performance test(s), was not reported and could not be estimated (e.g., in regard to dose, reporting the number of ‘puffs’ smoked from a cannabis cigarette was not considered adequate to estimate dose); (3) there was no confirmation of a ≥24-h abstinence period for intoxicating substances (e.g., cannabis, alcohol, other recreational drugs) before performance assessments. See eTable 3 for PICOS statement. Three authors (LL, LE, and AC) assessed study eligibility and quality blinded, and resolved any disagreement by consensus. Authors LL, CP, and LE screened titles and abstracts. Authors LL and AC assessed full texts for eligibility and quality.
Data extraction and outcome measures
Studies were required to have measured driving performance, a discrete cognitive skill (e.g., information processing), and/or subjective cognitive or psychomotor function. These performance outcomes were used as markers of potential impairment. Each cognitive test used in the included studies was categorized into a performance domain (Table 1). Categorizations of measures by cognitive function/domain were based on previous meta-analyses, to allow for greater comparability across the literature [9, 19]. All outcome measures of neurocognitive function or psychomotor performance on objective or subjective assessments were extracted for each domain (e.g., reaction time, accuracy of responses, mean score etc.). Additional variables were extracted including study participant characteristics, dose, product type, method of administration, concomitant drugs, comorbidities, cannabis experience, and type of performance assessment. The primary outcome was the peak mean difference in acute performance measures between CBD and placebo, as quantified by Hedges’ g. The secondary outcome was the peak mean difference in acute performance measures between CBD and Δ9-THC. Eligible effect estimates for the peak mean difference in studies with multiple time points were constrained to 0–120 minutes post-inhaled cannabis and 30–240 min post-oral cannabis consumption given the pharmacokinetics of each route of administration [13, 20, 21]. See eMethods for further details.
Effect size computation
Hedges’ g effect estimates were calculated from the standardized mean difference (SMD) between matched intervention groups (CBD, Δ9-THC, placebo). Hedges’ g was used to provide a more unbiased estimate for small sample sizes [22]. All effect sizes were recorded such that positive Hedges’ g values indicated a greater magnitude of impaired performance. Effect sizes were interpreted using the convention (g = 0.2 [small], 0.5 [medium], 0.8 [large]) [23]. In order to compute Hedges’ g, Cohen’s d was first computed using the formula [24]:
The standard deviation within groups was imputed from the standard deviation of the difference using the formula:
where r is the correlation between pairs of observations. If r was not reported or unable to be calculated from raw data, the standard 0.5 assumption was used. Cohen’s d was then converted to Hedges’ g using the formula:
The J conversion factor was computed using the formula:
Meta-analytic methods
Omnibus effect estimates and moderation analyses were conducted using a robust variance estimation (RVE) meta-regression approach. The RVE approach allows for the incorporation of dependent effect size measurements (e.g., multiple effect sizes from crossover studies or studies with multiple outcome measures for the same participants) without violating independence assumptions by using robust standard errors based on heteroskedasticity-robust estimates and clustered methods (see refs. [25, 26] for details). This analysis utilized a modified RVE method for small-sample size adjustments [26]. Moderator analyses were carried out for the primary outcome of peak mean difference between CBD and placebo in a series of one-covariate analyses. Model outputs were not interpreted if the degrees of freedom were <4, as recommended by Hedges et al., 2010.
Sensitivity analyses were conducted using a standard random-effects meta-regression approach. In studies with multiple effects, effect estimates were averaged to produce a single effect. Traditional publication bias measures (e.g., Egger’s plot for funnel asymmetry) were conducted on the average effect size model as they have not yet been widely validated for RVE models.
All analyses were carried out in R using the robumeta [27] and Metafor packages [28].
Risk of bias and quality assessment
All studies were assessed for risk of bias using the revised Cochrane Risk of Bias (RoB) tool [29]. The RoB 2.0 comprises five domains, including the randomization process, deviation from intended interventions, missing data, measurement of the outcome, selective outcome reporting, and “other sources of bias”. Two independent assessors (LL and AC) performed the risk of bias assessments, with any disagreement resolved by consensus. A decision around the interpretability of the available evidence was made by categorizing studies by the research question and rating them based on their quality.
Results
Study characteristics
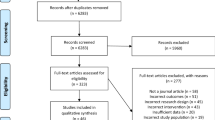
Figure 1 shows the PRISMA flowchart of study selection. Given the limited literature base, a broad search strategy was adopted in an attempt to capture all possible studies (See eMethods for more details). A total of 15,990 records were identified from database searches. After the removal of duplicates, 11,355 records were screened, of which 508 documents were reviewed for eligibility by full text. A total of 20 studies were included, where 16 studies were included in the quantitative analysis [12, 13, 30,31,32,33,34,35,36,37,38,39,40,41,42,43] and an additional four studies were included in the qualitative synthesis due to insufficient data for quantitative synthesis [44,45,46,47]. Among the 16 studies included in the quantitative analysis, there was minimal missing outcome data, with only one timepoint of an outcome missing in a single study. Additionally, supplementary and/or raw data were received from eight of the 16 studies.
The characteristics and key findings of the 20 included studies are presented in Table 2 and eTable 3. Outcome measures and dependent variables for each study included in the quantitative synthesis are presented in eTables 4 and 5. Seventeen studies were double-blind, randomized, placebo-controlled cross-over designs and three were double-blind, randomized, placebo-controlled parallel-group trials. Of the eligible studies, 14 included healthy adult participants [12, 13, 30,31,32,33,34,35,36, 38, 39, 42, 46, 47]; one study included adults with social anxiety disorder [44]; one study was comprised of participants with psychosis [41]; one study was comprised of participants at high-risk of psychosis [45]; one study was participants with nicotine dependence [37]; one study included healthy adults with low and high Schizotypy Personality Questionnaire scores, but no clinically diagnosed schizophrenia or psychosis [40]; and one study included adults with chronic pain and fibromyalgia [43]. The majority of study populations were cannabis-naive or had few lifetime exposures. Only three studies included participants who had a recent history of occasional or frequent cannabis use [40, 42, 45]. Cannabinoids were primarily administered through an oral route (N = 14, 70%) or via vapourization (N = 5, 25%) alone, with one study administering both oral and vapourized cannabinoids (N = 1, 5%) [13]. Doses of oral CBD ranged from 15 mg to 4500 mg and from 12.5 to 400 mg for vapourized CBD. Doses of oral Δ9-THC ranged from 10 mg to 30 mg and vapourized doses ranged from 8 mg to 30 mg Δ9-THC. All included studies used a single-dose regimen. Qualitative findings are presented in the eResults.
Quantitative findings
Omnibus meta-analysis of peak performance effects of acute CBD exposure compared to placebo
The omnibus RVE meta-analysis indicated a significant, but small effect size for impaired performance following acute CBD consumption compared to placebo (Hedges’ g = 0.122, 95% CI: 0.023–0.221, p = 0.019). Moderate heterogeneity was observed among studies (I2 = 38.24%). A consistent omnibus estimate was observed when collapsing effect sizes into a single average estimate for each study, Hedges’ g = 0.113, 95% CI: 0.014–0.212, p = 0.026. Model results are presented in Table 3.
Moderator analyses
Results of moderator analyses are presented in Table 3. A significant moderator effect was observed for measure type. This effect reflected larger mean differences between CBD and placebo when subjective measures were used (Hedges’ gSubjective = 0.288 versus Hedges’ gObjective = 0.048). Objective measures were not found to be significantly different than 0 when changed to the reference group to test the significance of the intercept.
A significant moderation effect was observed for cognitive function. This reflected a significantly larger mean difference for measures of subjective sedation/tiredness (Hedges’ g = 0.329) compared to episodic memory (Hedges’ g = 0.066) and working memory (Hedges’ g = 0.026). Information processing measures were not observed to have a significantly different mean difference compared to subjective sedation/tiredness measures (p = 0.157). However, only subjective sedation/tiredness was observed to be significantly different from 0. Comparisons to measures of divided attention, driving, executive function and subjective alertness could not be made due to insufficient degrees of freedom.
CBD dose and route of administration were not significant moderators (ps > 0.5).
Comparisons to Δ9-THC
Two secondary analyses were conducted to assess the difference in peak performance effect between CBD and Δ9-THC (Table 4). The first analysis compared peak mean difference in performance measures between cannabis and placebo, with cannabinoid (CBD or Δ9-THC) as a moderator. Eight studies that had a CBD and a Δ9-THC arm were included. Cannabinoid type was a significant moderator of effect sizes. This effect reflected larger mean differences between Δ9-THC and placebo (Hedges’ g = 0.356, 95% CI: 0.059–0.398, p = 0.016) compared to CBD and placebo (Hedges’ g = 0.128).
The second analysis provided a direct comparison of the peak mean difference in performance measures between Δ9-THC consumption compared to CBD consumption. Eight studies that had a CBD and Δ9-THC arm were included. The omnibus RVE meta-analysis indicated a significantly greater effect on performance for Δ9-THC compared to CBD. This effect reflected a moderate effect size for impaired performance following Δ9-THC consumption compared to CBD (Hedges’ g = 0.416, 95% CI: 0.017–0.816, p = 0.043).
Quality of evidence
The quality of available evidence was deemed moderate-to-high (See eFig. 1 and eResults). Of the 20 clinical trials analyzed, three (15%) were deemed to have an overall ‘low risk’ of bias, 16 (76%) were assessed as having ‘some concerns’, and one (5%) was identified as ‘high risk’ of bias.
Egger’s test for funnel plot asymmetry was not statistically significant, consistent with the funnel plot visual (See eFig. 2 and eResults).
Discussion
The results of this meta-analysis indicate that acute CBD consumption had a small but statistically significant effect on performance as assessed by all outcomes in aggregate, compared to placebo. Moderator analyses revealed this effect was significant only for subjective ratings of sedation/drowsiness, and no significant effects were observed for objective task performance on domains including memory, psychomotor ability, driving performance, information processing, attention, or higher order cognitive functioning. Dose and route of administration were not significant moderators in this analysis. As expected, acute doses of Δ9-THC produced significantly greater impaired performance than CBD relative to placebo and in direct comparison to CBD under the same experimental conditions. It is important to note that this sample was composed of primarily naive or infrequent cannabis users. It is unknown if these findings would translate to individuals with consistent cannabis product use, generally, or CBD use, specifically (e.g., medical cannabis patients). Additionally, the small, statistically significant effect size for the primary comparison of performance on cognitive and psychomotor measures between CBD to placebo may not translate to functional impairment, particularly given that these differences were limited to subjective feelings of sedation or tiredness.
This evidence synthesis supports that acute CBD consumption does not negatively impact neurocognitive function, as assessed by objective neurocognitive measures, consistent with findings from earlier trials and reviews [11, 48, 49]. It is important to note that these findings are from a sample of primarily infrequent cannabis consumers and may not represent the actual population of individuals who use CBD chronically. Infrequent cannabis consumers would most likely have the highest risk of impairment compared to individuals who use CBD chronically. Additionally, this sample was primarily in healthy adults. The effect of CBD may be different in different clinical populations. The small effect of subjective sedation noted in the current study has been reported inconsistently within previous literature. Somnolence and sedation are noted as potential side effects in Epidiolex prescribing information [50]. However, it has been proposed that CBD-related sedation in the context of the treatment of epilepsy may be due to drug interactions rather than CBD itself [51, 52].
Discrepancies between subjective and objective indicators of impairment have been noted previously. Some evidence suggests that people who use cannabis may overestimate their level of sedation and other indicators of impairment [53, 54], while others may compensate for expected impairment-related effects [55]. Drug expectancy may also contribute to this phenomenon. The expectation of receiving a certain drug can produce subjective and behavioral effects similar or opposite to those related to the drug, even in the absence of the drug itself. Such expectations can be formed by verbal information about the content and supposed effects of the drug, prior experience, and observational learning [56]. Metrik et al. [55, 57] have shown that the expectancy of receiving Δ9-THC produces greater subjective effects, including euphoria and sedation. CBD expectancy may also impact subjective and drug effect ratings [58]. Given that cannabis expectancy seems to affect self-reported reactions and drug responses, this calls into question the level of functional impairment associated with the small effect size obtained from this synthesis.
As expected, Δ9-THC produced significantly higher magnitudes of impaired performance compared to CBD. This adds validation for detecting and examining impaired cognitive and psychomotor performance for CBD and THC using the same experiments and designs. However, the question of whether concurrent CBD and Δ9-THC consumption increases or decreases the magnitude of impairment remains. Many cannabis products contain both CBD and Δ9-THC, including whole-plant CBD-dominant products. Additionally, CBD may be co-administered with Δ9-THC preparations with the expectation that CBD can ameliorate Δ9-THC-related cognitive impairment, anxiety, and sedation while also offering a range of therapeutic benefits [59,60,61]. Evidence from both experimental and naturalistic studies suggest that the addition of CBD to Δ9-THC produces differential dose-dependent effects, which may depend on the ratio of CBD:Δ9-THC and route of administration [30, 42, 43, 62, 63]. One study found that low-dose vapourized CBD (4 mg) enhanced impairment relative to Δ9-THC (8 mg) alone, whereas high-dose CBD (400 mg) reduced impairment across objective and subjective measures [42]. Other studies have reported that vapourized Δ9-THC/CBD-equivalent cannabis (13.75 mg Δ9-THC + 13.75 mg CBD) is no less impairing than Δ9-THC-dominant cannabis (13.75 mg Δ9-THC), and in some cases CBD may actually exacerbate Δ9-THC-induced acute impairment, as measured by psychomotor assessments and simulated driving performance [47, 62]. Pharmacokinetic data from the available research has also shown that peak plasma concentrations of Δ9-THC appear to be higher when CBD is co-administered [30, 43, 62], although several studies have also found no evidence of changes [64,65,66]. CBD can inhibit the metabolism of Δ9-THC and other drugs, and these interactions are more likely to occur after oral ingestion of CBD than with inhalation [67,68,69,70]. Thus, it is imperative to consider the potential for CBD to increase impairment when combined with other drugs, even if acute doses of CBD alone are not associated with functional impairment in controlled research studies.
The majority of participants in the current investigation were naive to cannabis or had few lifetime exposures. It has previously been observed that people who regularly use cannabis experience less cannabis-associated impairment compared to those with occasional use [9, 71]. As such, it is unknown how these findings would translate to populations with more frequent cannabis use (e.g., medical cannabis patients). However, it could be predicted that the small effect on subjective sedation observed in the current study may be diminished with frequent CBD use, in line with what has been observed in studies assessing Δ9-THC-associated impairment in frequent cannabis users [9, 72]. Further, some evidence suggests that CBD may improve cognitive function with prolonged use [73].
Future directions
The available literature on the acute performance effects of CBD consumption only allowed for assessment of performance in certain domains of cognitive function and in certain contexts of use (e.g., naive to cannabis consumption). Of key importance, there is a need to examine the impact of frequent, long-term CBD use on neurocognitive function to examine if tolerance diminishes the observed effect. Particularly for common safety sensitive tasks completed by the general population, such as driving, to gain a more robust picture of real-world risk. Finally, the majority of the trials in this study used CBD isolate products. In the real-world, full spectrum CBD-dominant products (which include other major and minor cannabinoids [including low levels of Δ9-THC] and terpenes), balanced CBD:Δ9-THC products, and lower CBD to Δ9-THC ratio products are commonly used. Effects on neurocognitive performance associated with these products should be further investigated as other cannabinoids and terpenes may contribute to impairing effects.
Limitations
This meta-analysis had several limitations. There was insufficient data, due to the sparse number of studies that included frequent cannabis users, to examine the potential difference between infrequent and frequent cannabis users. As such, these findings may not translate to populations who consistently use CBD. Additionally, although moderation analyses were conducted to assess variability, there are undoubtedly other variables that may impact an individual’s magnitude or risk of impaired neurocognitive performance (e.g., comorbidities, concomitant medications) that were not addressed in the included studies.
Conclusion
This meta-analysis suggests acute CBD consumption may be associated with a small increase in subjective sedation compared to placebo in infrequent cannabis users, but does not significantly impact performance across a range of cognitive domains. These results are consistent with previous evidence supporting that CBD consumption does not impact neurocognitive function. As such, acute use of CBD in the absence of THC or other drugs is unlikely to lead to functional impairment. Further research is warranted to investigate the risk of impaired neurocognitive function with daily CBD consumption, in addition to assessing performance in alternative domains.
Data availability
Data related to this manuscript will be made available upon request.
References
Wadsworth E, Craft S, Calder R, Hammond D. Prevalence and use of cannabis products and routes of administration among youth and young adults in Canada and the United States: a systematic review. Addict Behav. 2022;129:107258.
Rotermann M. Looking back from 2020, how cannabis use and related behaviours changed in Canada. Health Rep. 2021;32:3–14.
Carnide N, Nadalin V, Mustard C, Severin CN, Furlan AD, Smith PM. Cannabis use among workers with work-related injuries and illnesses: results from a cross-sectional study of workers’ compensation claimants in Ontario, Canada. BMJ Open. 2023;13:e072994.
United Nations Office on Drugs and Crime. UNODC World Drug Report 2022 highlights trends on cannabis post-legalization, environmental impacts of illicit drugs, and drug use among women and youth. United Nations: Office on Drugs and Crime. 2021. www.unodc.org/unodc/en/frontpage/2022/June/unodc-world-drug-report-2022-highlights-trends-on-cannabis-post-legalization--environmental-impacts-of-illicit-drugs--and-drug-use-among-women-and-youth.html. Accessed 3 January (2024).
Wilson-Poe AR, Smith T, Elliott MR, Kruger DJ, Boehnke KF. Past-year use prevalence of cannabidiol, cannabigerol, cannabinol, and Δ8-tetrahydrocannabinol among US adults. JAMA Netw Open. 2023;6:e2347373.
Goodman S, Wadsworth E, Schauer G, Hammond D. Use and perceptions of cannabidiol products in Canada and in the United States. Cannabis Cannabinoid Res. 2022;7:355–64.
Arnold JC, Nation T, McGregor IS. Prescribing medicinal cannabis. Aust Prescr. 2020;43:152–9.
Han BH, Palamar JJ. Trends in Cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med. 24 February 2020. https://doi.org/10.1001/jamainternmed.2019.7517.
McCartney D, Arkell TR, Irwin C, McGregor IS. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: a systematic and meta-analytic review. Neurosci Biobehav Rev. 2021;126:175–93.
Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend. 2006;85:114–22.
Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–54.
Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–71.
Spindle TR, Cone EJ, Goffi E, Weerts EM, Mitchell JM, Winecker RE, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211:107937.
Hinton D, Asher T. Organizational policies addressing cannabidiol use in the workplace. Int J Appl Technol Leadersh. 2022;1:1–28.
Levitt H, Sheikh S. Cannabis Is Still a Hazy Workplace Issue in Canada, Years After Legalization. SHRM. 2022. https://www.shrm.org/resourcesandtools/hr-topics/global-hr/pages/canada-cannabis-years-after-legalization.aspx. Accessed 22 September (2023).
MacCallum CA, Lo LA, Boivin M. “Is medical cannabis safe for my patients?” A practical review of cannabis safety considerations. Eur J Intern Med. 2021;89:10–14.
MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav. 2018;80:154–60.
Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, et al. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. 2017;41:83–99.
Zamarripa CA, Vandrey R, Spindle TR. Factors that impact the pharmacokinetic and pharmacodynamic effects of cannabis: a review of human laboratory studies. Curr Addict Rep. 2022;9:608–21.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9.
Borenstein M, editor. Introduction to meta-analysis. Chichester, U.K: John Wiley & Sons; 2009.
Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1:39–65.
Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015;20:375–93.
Fisher Z, Tipton E. robumeta: An R-package for robust variance estimation in meta-analysis. arXiv. 2015;1503.02220.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Soft. 2010;36:1–48.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG. Effect of cannabidiol and Δ 9 -tetrahydrocannabinol on driving performance: a randomized clinical trial. JAMA. 2020;324:2177.
Arout CA, Haney M, Herrmann ES, Bedi G, Cooper ZD. A placebo-controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br J Clin Pharm. 2022;88:347–55.
Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–51.
Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis byΔ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012;69:27–36.
Bloomfield MAP, Green SF, Hindocha C, Yamamori Y, Yim JLL, Jones APM, et al. The effects of acute cannabidiol on cerebral blood flow and its relationship to memory: an arterial spin labelling magnetic resonance imaging study. J Psychopharmacol. 2020;34:981–9.
Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–73.
Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology. 1979;66:45–50.
Hindocha C, Freeman TP, Grabski M, Crudgington H, Davies AC, Stroud JB, et al. The effects of cannabidiol on impulsivity and memory during abstinence in cigarette dependent smokers. Sci Rep. 2018;8:7568.
Hotz J, Fehlmann B, Papassotiropoulos A, De Quervain DJF, Schicktanz NS. Cannabidiol enhances verbal episodic memory in healthy young participants: a randomized clinical trial. J Psychiatr Res. 2021;143:327–33.
McCartney D, Suraev AS, Doohan PT, Irwin C, Kevin RC, Grunstein RR, et al. Effects of cannabidiol on simulated driving and cognitive performance: a dose-ranging randomised controlled trial. J Psychopharmacol. 2022;36:1338–49.
Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, Curran HV. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry. 2018;8:181.
O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2021;51:596–606.
Solowij N, Broyd S, Greenwood L, Van Hell H, Martelozzo D, Rueb K, et al. A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci. 2019;269:17–35.
van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160:860–9.
Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219–26.
Bhattacharyya S, Wilson R, Appiah-Kusi E, O’Neill A, Brammer M, Perez J, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75:1107.
Winton-Brown TT, Allen P, Bhattacharrya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an fMRI study. Neuropsychopharmacology. 2011;36:1340–8.
Woelfl T, Rohleder C, Mueller JK, Lange B, Reuter A, Schmidt AM, et al. Effects of cannabidiol and delta-9-tetrahydrocannabinol on emotion, cognition, and attention: a double-blind, placebo-controlled, randomized experimental trial in healthy volunteers. Front Psychiatry. 2020;11:576877.
Bergamaschi MM, Queiroz RHC, Zuardi AW, Crippa JAS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–49.
Brown JD, Winterstein AG. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8:989.
U.S. Food and Drug Administration. EPIDIOLEX Full Prescribing Information. 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf. Accessed 6 May (2023).
Franco V, Perucca E. Pharmacological and therapeutic properties of cannabidiol for epilepsy. Drugs. 2019;79:1435–54.
Landmark CJ, Brandl U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord. 2020;22:S16–S22.
Love S, Larue GS, Rowland B. Alignment of subjective and objective driving impairment following alcohol and cannabis use: a systematic review. Appl Cogn Psychol. 2023;37:1167–82.
Robbe HWJ, O’Hanlon JF. Rijksuniversiteit Limburg. Marijuana and Actual Driving Performance. 1993.
Metrik J, Kahler CW, Reynolds B, McGeary JE, Monti PM, Haney M, et al. Balanced placebo design with marijuana: Pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology. 2012;223:489–99.
Kirsch I. Response Expectancy and the Placebo Effect. International Review of Neurobiology. 2018;138:81–93.
Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TAR, de Wit H, et al. Effectiveness of a marijuana expectancy manipulation: piloting the balanced-Placebo design for marijuana. Exp Clin Psychopharmacol. 2009;17:217–25.
Spinella TC, Stewart SH, Naugler J, Yakovenko I, Barrett SP. Evaluating cannabidiol (CBD) expectancy effects on acute stress and anxiety in healthy adults: a randomized crossover study. Psychopharmacology. 2021;238:1965–77.
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27.
Morgan CJA, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. 2012;42:391–400.
Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–46.
Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology. 2019;236:2713–24.
Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry. 2010;197:285–90.
Englund A, Oliver D, Chesney E, Chester L, Wilson J, Sovi S, et al. Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different CBD:THC ratios. Neuropsychopharmacology. 2023;48:869–76.
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk E-M, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810.
Pennypacker SD, Romero-Sandoval EA. CBD and THC: do they complement each other like Yin and Yang? Pharmacotherapy. 2020;40:1152–65.
Bansal S, Paine MF, Unadkat JD. Comprehensive predictions of cytochrome P450 (P450)-mediated in vivo cannabinoid-drug interactions based on reversible and time-dependent P450 inhibition in human liver microsomes. Drug Metab Dispos. 2022;50:351–60.
Bansal S, Zamarripa CA, Spindle TR, Weerts EM, Thummel KE, Vandrey R, et al. Evaluation of cytochrome P450-mediated cannabinoid-drug interactions in healthy adult participants. Clin Pharm Ther. 2023;114:693–703.
Bansal S, Ladumor MK, Paine MF, Unadkat JD. A physiologically-based pharmacokinetic model for cannabidiol in healthy adults, hepatically-impaired adults, and children. Drug Metab Dispos. 2023;51:743–52.
Zamarripa CA, Spindle TR, Surujunarain R, Weerts EM, Bansal S, Unadkat JD, et al. Assessment of orally administered Δ9-Tetrahydrocannabinol when coadministered with cannabidiol on Δ9-Tetrahydrocannabinol pharmacokinetics and pharmacodynamics in healthy adults: a randomized clinical trial. JAMA Netw Open. 2023;6:e2254752.
Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–77.
Eadie L, Lo LA, Christiansen A, Brubacher JR, Barr AM, Panenka WJ, et al. Duration of neurocognitive impairment with medical cannabis use: a scoping review. Front Psychiatry. 2021;12:638962.
Solowij N, Broyd SJ, Beale C, Prick J-A, Greenwood L, van Hell H, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3:21–34.
Acknowledgements
The authors would like to acknowledge Queen’s University librarian Sandra McKeown for her assistance in developing the search strategy.
Author information
Authors and Affiliations
Contributions
Concept and design: LAL, JCS, RV, and CAM; Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: LAL, ALC, and CAP. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: LAL and JCS. Administrative, technical, or material support: JCS, RV, and CAM. Supervision: JCS, RV, and CAM.
Corresponding author
Ethics declarations
Competing interests
LAL reports financial payment from Ontario Cannabis Store for health education services. JCS reports receiving research funding from Canopy Growth Corporation and the Cure Addiction Now foundation. RV is a paid consultant or advisory board member to MIRA1a Pharmaceuticals, Jazz Pharmaceuticals, Canopy Growth Corporation, Charlotte’s Web, Syqe Medical Ltd., and WebMD. CAM is the Medical Director of Greenleaf Medical Clinic. She was formerly on the Board of Directors for The Green Organic Dutchman. She is an advisor to PreveCeutical, Africanna, Andira Medicine, and Dosist. Additionally, she has provided medical consultation and/or received support for industry sponsored continuing medical education from: Aleafia, Aphria, Aurora, Canopy Growth Corporation, EO Care, Emerald Health and Syqe Medical. All other authors report no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lo, L.A., Christiansen, A.L., Strickland, J.C. et al. Does acute cannabidiol (CBD) use impair performance? A meta-analysis and comparison with placebo and delta-9-tetrahydrocannabinol (THC). Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01847-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01847-w