Abstract
The poor translatability between preclinical and clinical drug trials has limited pro-cognitive therapeutic development. Future pro-cognitive drug trials should use translatable cross-species cognitive tasks with biomarkers (1) relevant to specific cognitive constructs, and (2) sensitive to drug treatment. Here, we used a difficulty-modulated variant of a cross-species cognitive control task with simultaneous electroencephalography (EEG) to identify neurophysiological biomarkers sensitive to the pro-cognitive effects of dextroamphetamine (d-amp) (10 or 20 mg) in healthy adults (n = 23), in a randomized, placebo-controlled, counterbalanced, double blind, within-subject study, conducted across three test days each separated by one week. D-amp boosted d-prime, sped reaction time, and increased frontal P3a amplitude to non-target correct rejections independent of task difficulty. Task difficulty did however, moderate d-amp effects on EEG during target performance. D-amp suppressed frontal theta power during easy target responses which negatively correlated with drug-induced improvement in hit rate while d-amp-induced changes in P3b amplitude during hard target trials strongly correlated with drug-induced improvement in hit rate. In summary, d-amp affected both behavioral and neurophysiological measures of cognitive control elements. Under low-demand, d-amp diminished cognitive control by suppressing theta, yet under high-demand it boosted control in concert with higher P3b amplitudes. These findings thus appear to reflect a gain-sharpening effect of d-amp: during high-demand processes were boosted while during low-demand processes were neglected. Future studies will use these neurophysiological measures of cognitive control as biomarkers to predict d-amp sensitivity in people with cognitive control deficits, including schizophrenia.
Similar content being viewed by others
Introduction
Despite decades-long efforts to develop cognitive enhancing treatments for patients with neuropsychiatric disorders including, schizophrenia (SZ), no Food and Drug Administration-approved pro-cognitive drugs exist. Numerous pro-cognitive drug trials have been successful in animals and healthy humans, yet these drugs have failed to improve cognition in patients [1]. This failure of pro-cognitive drug trials has underscored the need to identify biomarkers of treatment sensitivity. To this end, several initiatives have sought to develop cross-species tests with tightly defined behaviors and neurobiological underpinnings [2, 3], to identify biomarkers that can be translated across species and predict treatment sensitivity.
As part of our ongoing studies of translational biomarkers, we developed several cross-species paradigms with consistent electroencephalogram (EEG)-based biomarkers of specific cognitive domains for use in both mice and humans [4]. One candidate domain was cognitive control, defined as the ability to optimally bias attention, perception, and actions, in the service of a mental and behavioral goal while inhibiting a prepotent response. We quantified cognitive control using a difficulty-modulated version of the reverse-translated cross-species 5-choice continuous performance test (5C-CPT; [5, 6]).
The validity of the 5C-CPT paradigm to measure cognitive control has been reported in humans, rats, and mice [7,8,9,10,11]. Importantly, the response inhibition component of the 5C-CPT enables cognitive control assessment, which is separable from the motoric impulsivity measure, premature responses [12]. Furthermore, the 5C-CPT can be combined with EEG recording to identify brain regions involved in different elements of the cognitive control construct, including the fronto-parietal brain regions that are critical for goal maintenance and updating, response inhibition and performance monitoring [13,14,15,16,17].
Previously, in a proof-of-concept study, we demonstrated that the catechol-O-methyltransferase inhibitor, tolcapone, enhanced the frontal P2 during correct non-target trials, and that this effect was directly correlated with tolcapone-reduced false alarm rate (FAR) in healthy subjects [18]. This finding was evident when these healthy subjects were stratified based on their 5C-CPT performance at baseline, yet this was obscured by ceiling levels of 5C-CPT performance. To raise this ceiling, other CPTs have incorporated visual discriminability, e.g., degraded stimulus CPT [19, 20], a memory component (e.g., CPT-Identical Pairs), or a working memory component (e.g., AX-CPT) [21, 22]. In the present study, we developed a ‘masking’ visual discrimination challenge to increase cognitive effort during the 5C-CPT target/non-target trials that remained selective to the cognitive control domain [23]. Here, we tested whether this new difficulty manipulation altered the pharmacological sensitivity of the EEG markers.
While the interpretation of EEG signals are continually defined, the 5C-CPT elicits some of the best-known EEG features of cognitive control. Frontal midline theta is elicited during demanding performance, and it is a reliable marker that signals the need for cognitive control [24]. The P3a is closely associated with the novelty-evoked instantiation of response selection [25], and P3b reflects later decision making [26,27,28,29,30]. Theta and P3a occur earlier and reflect a lower level of awareness than later decision variables like the P3b, which relies on slow and deliberative accumulation of available evidence leading to a decision [24, 29]. Both these event-related potential (ERP) components and frontal midline theta are sensitive to pharmacological interventions [31] and can serve as candidate biomarkers to predict treatment sensitivity. We chose dextroamphetamine (d-amp) as the pro-cognitive “test” drug because of its known mechanism of action, and well-established neurocognitive and neurophysiological cross-species effects [8].
Pharmacological studies using d-amp, a dopamine/norepinephrine transporter inhibitor and indirect dopamine agonist, reported improved performance on cognitive control-requiring tasks in both healthy controls and stable SZ patients [32,33,34]. D-amp also improved standard 5C-CPT performance in humans, mice, and rats by increasing hit rate (HR), signal detection, and speeding hit reaction time (RT) [7, 8]. However, d-amp effects on the EEG correlates of cognitive control remain unknown. We hypothesized that d-amp would improve this cross-species 5C-CPT performance by increasing HR (target detection) and speeding hit RT, as well as by enhancing frontal activation during correct rejections (CRs) on non-target trials and enhancing theta during difficult target trials. We also hypothesized that neural signals of cognitive control would correlate with relevant behavioral performance.
Materials and methods
This study was conducted at the UCSD Medical Center, with approval from the UCSD Human Subject Institutional Review Board.
Subjects: psychiatrically and medically healthy men and women between the ages 18–35 years were recruited from the community via public advertisements and compensated monetarily for study participation. First, subjects underwent phone screening to assess current and past medical and psychiatric history, medication and recreational drug use and family history of psychosis. Subjects who passed the phone screen were invited for a screen day. During the screening visit, subjects first signed the consent form and then completed the following assessments: (1) structured clinical interview (SCID-NP; [35]); (2) self-reporting questionnaires about caffeine intake and handedness; (3) a hearing test; (4) physical examination; (5) an electrocardiogram (EKG); (6) urine toxicology screen; (7) urine pregnancy test for females as per our established screening protocol [36]; (8) a Wide Range Achievement Test for IQ assessment [37]; and (9) a Measurement and Treatment Research to Improve Cognition in Schizophrenia Cognitive Battery [38]. Study inclusion criteria are described in Supplementary Table 1.
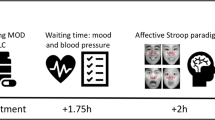
Study design: this study used a double blind, randomized, placebo-controlled, counterbalanced, within-subject design. Participants received either placebo or one of two active doses of d-amp (10 or 20 mg) orally on each of the three test days separated by 1 week. The test day schedule is shown in Supplementary Table 2. Briefly, subjects arrived at 8:30 a.m. after overnight fasting with exception of water, completed a urine toxicology screen and a urine pregnancy test in females, and ate a standardized breakfast. Vital signs and subjective symptom rating scale (SRS) scores [39] were obtained at specific intervals pre- and post-pill. Subjects completed the 5C-CPT with simultaneous EEG recording 120 min after pill administration, when these doses of d-amp are known to be bioactive [39, 40].
Masked 5-choice continuous performance task (5C-CPT) with simultaneous EEG recording
As reported previously [9], the participants were given a brief practice on the task before performing the full task. The practice session consisted of 12 trials (10 target and 2 non-target stimuli randomly presented). The participants had to perform the practice block correctly before moving on to the full task. For target trials, participants responded by moving the joystick in the direction of a circle that turned white one at a time, and they inhibited from responding when all 5 circles turned white simultaneously (non-target trials). We used a modified version of the 5C-CPT with two different conditions of target and non-target stimuli. Consistent with previous studies, unmasked condition or “easy” stimuli were presented for 100 ms, and masked condition or “hard” stimuli were presented for 10 ms. The stimuli presented in masked condition were identical in appearance to unmasked conditions, except that a solid white mask was presented over the stimulus array for 90 ms after initial stimulus presentation (100 ms total, consistent with standard trails; Fig. 1). All target and non-target stimuli, whether masked or unmasked, were presented in a pseudorandom order to ensure that no more than 3 of the same trial types appeared consecutively, with a 1 s response window available for all trials and a variable inter-trial interval (ITI; 500, 1000, or 1500 ms). All participants understood the task and correctly performed the practice block prior to initiating the session. The full task consisted of 216 trials, 108 unmasked (90 target and 18 non-target stimuli), and 108 masked (90 target and 18 non-target stimuli) trials.
Consistent with the mouse 5C-CPT, participants must respond to target trials (single circle), but inhibit from responding to non-target trials (five circles). Standard 100 ms stimulus duration trials are presented as the unmasked (easy; blue diamonds) condition, while masked (hard; red circles) conditions are presented where a solid white mask is presented over the stimulus array 10 ms after the initial stimulus, for 90 ms, for a total of 100 ms (A). The impact of d-amp (10 or 20 mg), on healthy participant performance of this masked challenge 5C-CPT was assessed. D-amp (10 mg), sped hit reaction time (RT) for hard trials (*p = 0.002; C), while both 10 (*p = 0.03), and 20 mg (*p = 0.05), d-amp increased overall performance (d prime) independent of trial type (D), driven by increased target detection (B), without affecting bias of responding (E). Data presented as mean ± standard error of mean (SEM).
Responses were recorded and included hits and misses to target trials, and false alarms (FAs) and CRs to non-target trials. Composite metrics of task performance were used in the analysis of performance, including HR, FAR, and task accuracy, hit RT as indicated in our previous work [11], but this time compiled by trial condition (unmasked or “easy” vs. masked or “hard”). The d-prime and responsivity indices (measures of vigilance and bias respectively) were also calculated using signal detection theory [41, 42], the former measures appropriate responding [43], the latter provides a measure of the tendency to respond (bias) for each difficulty condition.
EEG recording and pre-processing
Continuous EEG data were recorded in DC mode from 64 scalp leads using a BioSemi Active Two system (www.biosemi.com). During data acquisition the electrode offsets were kept below 25 mV and all channels were referenced to the system’s internal loop (CMS/DRL electrodes). Four electrooculograms recorded at the superior and inferior orbit of the left eye and outer canthi of each eye, and one nose and two mastoid electrodes were used for offline re-referencing. All data were collected using a 1048 Hz sampling rate utilizing a first-order anti-aliasing filter. Data were epoched around the imperative stimuli, average referenced, and down-sampled to 500 Hz. Bad channels and bad epochs were identified using a conjunction of the FASTER algorithm [44] and pop_rejchan from EEGLab [45] and were subsequently interpolated and rejected, respectively. Eye blinks were removed following independent components analysis in EEGLab.
EEG post-processing
Time frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets defined as a Gaussian-windowed complex sine wave: ei2πtfee−t^2/(2 × σ^2), where t is time, f is frequency (which increased from 1–50 Hz in 50 logarithmically spaced steps) and the width (or “cycles”) of each frequency band were set to increase from 3/(2πf) to 10/(2πf) as frequency increased. Then, the time series was recovered by computing the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in estimates of instantaneous power taken from the magnitude of the analytic signal. Each epoch was then cut in length (stimuli: −500 to +1000 ms; responses: −1000 to +500 ms). Averaged power was normalized by conversion to a decibel (dB) scale (10 × log10[power(t)/power(baseline)]), allowing a direct comparison of effects across frequency bands. The baseline consisted of averaged power −300 to −200 ms before all stimuli. A 100 ms duration is often used as an effective baseline since pixel-wise time frequency data points have already been resolved over smoothed temporal and frequency dimensions with the wavelets.
Statistical differentiation followed an a priori approach, where each task had a predicted ERP component or temporal and frequency range for the contrast of interest. The time frequency region of interest was defined by a prior study of this same task [4]: response-locked theta power: 4–8 Hz from −500 to 0 ms pre-response. The time-locked event for non-target condition was set to the end of RT deadline. These data are shown in Supplementary section as they provide important analogs for future translational investigations but were not statistically analyzed. Stimulus-locked theta power was also investigated for the first time here (3–5.5 Hz from 200 to 400 ms poststimulus).
ERP components were quantified based on specific hypotheses. For non-target conditions, components were quantified as the average activity at the fronto-central midline scalp electrode (FCz) in a temporal window around the peak (P3a: 400 ms ± 50). For target conditions, the P3b (450 ms ± 100 ms) was quantified as the average activity at the parietal midline scalp electrode (Pz). These electrodes were chosen in-part due to our earlier biomarker observations in humans, and enabling future mouse testing at these same locations as we described [4].
Statistical analysis
Mixed linear models (MLMs) were run using MIXED command in SPSS 26 to analyze individual differences in 5C-CPT performance and EEG measures across drug conditions. MLMs used restricted maximum likelihood estimation with fixed effects for task difficulty (binary: easy, hard), drug (continuous: placebo, 10 mg, 20 mg), and the difficulty*drug interaction. Participants were modeled with random intercepts and the default diagonal covariance matrix. Correlations between individual differences on 5C-CPT behavioral performance and neural signals across drug conditions were analyzed using Pearson’s correlation. Alpha for all hypotheses was set at 0.05. Here we report linear trends, which had the best fit to the data. We also report on quadratic models in the Supplementary Material; conclusions were very similar to linear fits.
Results
Subjects
Of the 36 healthy participants enrolled in the study, 23 participants (Table 1) completed all three test days. Those disqualified from the study are shown in Supplementary Table 3. Study participants were mostly young, college educated men and women. D-amp was well tolerated except one study participant experienced elevated heart rate and blood pressure that lasted for more than 8 h after pill administration before resolving. Out of precaution, this participant was subsequently excluded from the study.
Physiological and psychological effects
D-amp significantly increased heart rate and blood pressure but had no significant effects on subjective ratings of fear, drowsiness or happiness measured using the SRS. For details see Supplementary Material.
5C-CPT behavior
For hit RT, there were significant main effects of d-amp (F1,52.36 = 11.06, p = 0.002; Fig. 1B) and task difficulty (F1,46.44 = 16.98, p < 0.001), but no drug*difficulty interaction (F < 1). Post hoc t-test contrasts revealed a significant effect of d-amp 10 mg dose (p = 0.007) compared to placebo for hard target trials. There was neither a significant effect of 20 mg d-amp compared to placebo nor differences between the two d-amp doses. There was a significant main effect of drug for d prime (F1,70.46 = 4.23, p = 0.044; Fig. 1C), but no significant effect of difficulty or drug*difficulty interaction (F’s < 1). Post hoc comparisons revealed significant differences between 10 mg (p = 0.03) and 20 mg d-amp dose (p = 0.057) compared to placebo but no difference between the two d-amp doses. There were no main or interaction effects for response bias, nor for independent measures of hits or FA (Fig. 1D, E and Supplementary Fig. 1). In summary, the masked 5C-CPT trials were significantly more difficult than the standard trials, while d-amp sped RT and increased signal detection irrespective of trial type.
Masked 5C-CPT related ERPs
Our ERP analysis was focused on frontal central (FCz), and parietal (Pz) electrodes given the consistency of effects across mice and humans in our earlier 5C-CPT work [4], confirming our a priori hypotheses here. There was a significant main effect of drug on non-target (CR) P3a (F1,59.64 = 16.89, p < 0.001), where d-amp increased P3a amplitude at FCz regardless of task difficulty (Fig. 2).
Grand average ERP waveforms on the top and topographic contrasts between d-amp 10 and 20 mg doses and placebo at the bottom for both easy (A) and hard (B) non-target trials. The FCz electrode site is identified on topographic plots with a magenta square. C ±Standard error of mean (SEM) plot showing d-amp increases in P3a amplitude at FCz during correct response inhibition (non-target trials), regardless of task difficulty (**p < 0.001).
This finding remained significant when the P3a was quantified as the difference between the P3a peak and the N2 trough (F1,64.70 = 15.43, p < 0.001). There were no significant effects for target (hit) P3b (difficulty F1,42.05 = 2.20, p = 0.15, drug or difficulty*drug interaction F < 1; Fig. 3A).
No effect of d-amp on P3b at Pz electrode for easy and hard target trials seen on grand average ERP waveform (A), topographic plot (B) and ±SEM plot (C). The Pz electrode site on topographic plot is identified with a magenta square. D d-amp-induced increases in hit rate directly correlated with d-amp-enhanced P3b during hard trials across three different contrasts: 10 mg-placebo, 20 mg-placebo, and 20 mg–10 mg (p = 0.04, p = 0.01, and p = 0.05, respectively) within subjects.
We investigated how drug-induced changes in performance related to drug-induced changes in ERPs. Due to trial count limitations, this analysis was restricted to correct target (hit) trials and the associated P3b component. Drug-induced increases in HR significantly correlated with drug-induced increases in P3b amplitudes on hard target trials across three different contrasts: 10 mg-placebo, 20 mg-placebo, and 20 mg–10 mg (p = 0.04, p = 0.01, and p = 0.05, respectively; Fig. 3B).
Theta power
There was no significant effect of d-amp on stimulus-locked theta power. However, analyses of response-locked theta power for target trials detected a significant difficulty*drug interaction (F1,72.33 = 5.95, p = 0.02) in the absence of main effects (Fs < 1; Fig. 4A, B). Planned comparisons split by task difficulty showed a significant decrease in theta power with increasing d-amp dose for easy target responses (F1,34.26 = 8.34, p = 0.007), but the corresponding increase in power with increasing d-amp dose for hard target responses was not significant (F1,29.59 = 1.51, p = 0.23). We investigated how drug-induced changes in performance related to drug-induced changes in response-locked theta power. Again, this analysis was restricted to correct target (hit) trials and the associated theta activity on easy (hit) responses. Drug-induced increases in HR were significantly correlated with drug-induced increases in response-locked theta power on easy target trials across two of the three different contrasts: 10 mg-placebo, 20 mg-placebo and 20 mg–10 mg (p = 0.01, p = 0.02 and p = 0.08, respectively; Fig. 4C). No such correlations were observed during hard target trials (Supplementary Fig. 3).
A Time frequency plots of response-locked target easy and hard trials and contrasts. The magenta box shows tf-ROI. B Line plots show d-amp induced theta suppression during easy target trials (**p = 0.007), not significant for hard trials but C. d-amp-induced increases in hit rate directly correlated with response-locked theta increases during easy target trials.
Discussion
This study investigated the acute effects of d-amp on the behavioral phenotypes and neural mechanisms of cognitive control using a cross-species reverse-translated masked challenge 5C-CPT task with simultaneous EEG recording in healthy adults. Both 10 and 20 mg doses of d-amp significantly enhanced 5C-CPT performance by increasing d prime and reducing hit RT. D-amp also activated frontal brain regions during CRs on non-target trials and suppressed frontal theta power during easy target responses. Interestingly, a series of significant correlations were noted between: (1) d-amp-enhanced HR and enhanced parietal P3b amplitude during hard target trials; and (2) d-amp induced frontal theta suppression and reduced HR during easy target responses. These intra-individual changes in parietal P3b amplitude and frontal theta suppression could serve as biomarkers for future pro-cognitive control drug trials in patient populations and mechanisms underlying these changes could be tested in rodents.
Importantly, both 10 and 20 mg doses of d-amp were well tolerated and biologically active, transiently increasing heart rate, systolic and diastolic blood pressure. These findings are consistent with prior reports of d-amp effects in healthy participants [36]. Our findings of reduced hit RT and enhanced signal detection with d-amp are also consistent with prior reports of d-amp in healthy adults and rodents [7, 8]. While d-amp sped responses for both easy and hard target trials, the hit RT was slower overall for hard target trials compared to easy target trials, likely reflecting increased attentional difficulty due to masking. This slowed RT likely enabled comparable HRs between the two conditions, suggesting that while masked trials were more challenging, they remained perceptible, and drove a speed/accuracy trade-off.
While the effect of d-amp on hit or FAR was not statistically significant, d-amp enhanced overall attention as measured by d-prime. Similar findings of d-amp effects on cognitive control measures using Connor’s CPT were reported in healthy adults [46]. The lack of significant effect on response inhibition could in part be due to the fewer number of non-target trials and sparse FA reflecting a ceiling effect. It could also suggest that d-amp is sensitive to attentional measures and vigilance, given its primary striatal action, but less sensitive to the behavioral measure of inhibitory control. However, Ilieva and colleagues [47] in their meta-analysis of 48 studies found that stimulants significantly improved inhibitory control in non-clinical individuals when measured using Go/NoGo or Stop Signal task, both of which include higher numbers of non-target trials. Increasing the NoGo (non-target) ratio (vs. target trials), in the 5C-CPT could enable greater sensitivity of this task to observe stimulant-induced effects on inhibitory outcomes. Given that we observed tolcapone-induced improved response inhibition and stronger P200 amplitude (which significantly correlated) previously in the standard 5C-CPT [18], we are confident this task is sensitive to drug-induced changes to inhibitory control. Thus, we continue efforts toward identifying treatment sensitive neurophysiological markers relevant to behavior.
Interestingly, we found that d-amp significantly activated frontal P3a during correctly rejected non-target trials independent of task difficulty. The P3a is associated with the orienting of response to novel or cognitively demanding events known to originate from frontal brain region and driven by dopamine [29, 48, 49]. It has also been linked to the control evoked by the need for response inhibition [25, 50]. Enhancement of the P3a suggests that one mechanism by which d-amp contributes to better performance (higher d-prime) is by facilitating response inhibition and target detection. The engagement and activation of appropriate cognitive control neural correlates of response inhibition in our study suggest a greater d-amp sensitivity of neurophysiological vs. behavioral measures of response inhibition, underscoring the potential value of neurophysiological biomarkers of treatment sensitivity.
Additionally, we found strong and reliable intra-individual effects (i.e., statistical random effects) between d-amp- enhanced HR and increased P3b amplitude during hard target trials (but not during easy or unmasked target trials). The P3b is a well-known marker of the meaning‐ and surprise‐driven updating of cognitive schema [26,27,28,29], particularly when integrating evidence toward a decision [51,52,53]. Our findings therefore suggest that P3b amplitude is a marker of the process by which d-amp boosts rapid and effective decision making under difficult target detection conditions (but not during easy or unmasked target trials)
D-amp suppressed frontal theta power during easy target responses. Moreover, individual differences in d-amp-induced theta suppression on easy target trials positively correlated with reduced HR on easy target trials. These findings stand in contrast to the facilitatory interpretations of enhanced P3a and P3b amplitudes: the main effect of diminished theta power (Fig. 4B) appears to be related to poorer performance across individuals (Fig. 4C). This thus appears to reflect a gain-sharpening consequence of d-amp: more critical processes were boosted while more simple processes were neglected. Inhibition (P3a) and decision (P3b) processes were enhanced in the service of better performance particularly on hard trials, whereas control over simple responses (theta) on easy trials was diminished to the detriment of performance. Thus, although d-amp boosted d-prime and RT, better performance came at a cost-generic performance monitoring processes appear to be diminished when they are least needed.
We previously reported response-locked theta power for target and non-target trials for both easy and hard stimuli (non-masked), in mice using a rodent 5C-CPT paradigm with simultaneous EEG monitoring [4]. Here, we report similar response-locked theta power with modified 5C-CPT in healthy adults (Supplementary Fig. 2). Thus, not only do these findings provide replicability of this biomarker in healthy human subjects, but they also support the cross-species translatability of the 5C-CPT paradigm and validate a task-relevant neural response.
While our study sample is modest, this limitation is mitigated somewhat by the use of a powerful within-subject design for dose comparisons across behavioral and brain-based measures. Although we were able to analyze individual dependent measures, our design lacked the power needed to use complex statistical approaches to identify multivariate relationships and latent factors that might moderate d-amp sensitivity.
In summary, d-amp significantly enhanced neural correlates of cognitive control construct in addition to behavioral measures of attention and vigilance in healthy adults using a modified cross-species 5C-CPT paradigm with simultaneous EEG recording. These findings demonstrate the feasibility of the study design and the use of a cross-species paradigm with simultaneous EEG recording and validates the behavioral and task-relevant EEG measures and their translatability across species. The scalp-recorded EEG activity reveals a mixture of frontal orienting/salience detection and parietal evaluative stimulus processing subcomponents noted as d-amp-enhanced frontal P3a during non-target trials and frontal theta suppression during easy target trials. Interestingly, the strong correlation between d-amp-induced frontal theta suppression and HR during easy target conditions, and the relationship between parietal P3b amplitude and d-amp-enhanced HR during difficult target conditions reflects a non-orthogonal model, wherein co-occurring processes combine in a weighted fashion depending on the task difficulty. In other words, d-amp contributes to a diminishment of control when the task is easy but promotes a tighter link between brain and behavior when the task is difficult or requires resolution of conflict. Future studies will focus on using these neurophysiological measures of cognitive control construct as biomarkers to predict pro-cognitive control drug sensitivity in patient populations, as well as mechanistic studies in rodents.
Data availability
All data and Matlab code to re-create these analyses are available at OpenNeuro.org, accession #[WILL BE COMPLETED UPON ACCEPTANCE].
References
Harvey PD, Bowie CR. Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am. 2012;35:683–98. https://doi.org/10.1016/j.psc.2012.06.008.
Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–37. https://doi.org/10.1093/schbul/sbm081.
National Institute of Mental Health. Research domain criteria. 2013. http://www.nimh.nih.gov/research-priorities/rdoc/nimh-researchdomain-criteria-rdoc.shtml.
Cavanagh JF, Gregg D, Light GA, Sharp RF, Bismark AW, Bhakta SG, et al. Electrophysiological biomarkers of behavioral dimensions from cross-species paradigms. Transl Psychiatry. 2021;11:482. https://doi.org/10.1038/s41398-021-01562-w.
Cope ZA, Young JW. The five-choice continuous performance task (5C-CPT): a cross-species relevant paradigm for assessment of vigilance and response inhibition in Rodents. Curr Protoc Neurosci. 2017;78:9.56.1–18. https://doi.org/10.1002/cpns.20.
Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: control of attention. Neurosci Biobehav Rev. 2013;37:2099–110. https://doi.org/10.1016/j.neubiorev.2012.05.009.
Young JW, Roberts BZ, Breier M, Swerdlow NR. Amphetamine improves rat 5-choice continuous performance test (5C-CPT) irrespective of concurrent low-dose haloperidol treatment. Psychopharmacology. 2020;237:1959–972. https://doi.org/10.1007/s00213-020-05511-1.
MacQueen DA, Minassian A, Kenton JA, Geyer MA, Perry W, Brigman JL, et al. Amphetamine improves mouse and human attention in the 5-choice continuous performance test. Neuropharmacology. 2018a;138:87–96. https://doi.org/10.1016/j.neuropharm.2018.05.034.
Bhakta SG, Young JW. The 5-choice continuous performance test (5C-CPT): a novel tool to assess cognitive control across species. J Neurosci Methods. 2017;292:53–60.
Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009;4:e4227. https://doi.org/10.1371/journal.pone.0004227.
Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behavi Brain Res. 2013;240:119–33. https://doi.org/10.1016/j.bbr.2012.11.028.
Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–92. https://doi.org/10.1016/j.bbr.2011.03.054.
Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. https://doi.org/10.1016/s0896-6273(03)00466-5.
Doege K, Kumar M, Bates AT, Das D, Boks MP, Liddle PF. Time and frequency domain event-related electrical activity associated with response control in schizophrenia. Clin Neurophysiol. 2010;121:1760–71. https://doi.org/10.1016/j.clinph.2010.03.049.
Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res. Cogn Brain Res. 2000;9:103–9. https://doi.org/10.1016/s0926-6410(99)0009-4.
Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. https://doi.org/10.1038/35036228.
Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–40. https://doi.org/10.1016/jbandc2004.09.016.
Bhakta SG, Light GA, Talledo JA, Balvaneda B, Hughes E, Alvarez A, et al. Tolcapone-enhanced neurocognition in healthy adults: neural basis and predictors. Int J Neuropsychopharmacol. 2017;20:979–87. https://doi.org/10.1093/ijnp/pyx074.
Parasuraman R, Mutter SA, Molloy R. (1991). Sustained attention following mild closed-head injury. J Clin Exp Neuropsychol. 1991;13:789–811. https://doi.org/10.1080/01688639108401090.
Mass R, Wolf K, Wagner M, Haasen C. Differential sustained attention/vigilance changes over time in schizophrenics and controls during a degraded stimulus continuous performance test. Eur Arch Psychiatry Clin Neurosci. 2000;250:24–30. https://doi.org/10.1007/pI0000753.
Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. Am J Med Genet. 2000;97:52–7. 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6.
Barch DM, Berman MG, Engle R, Jones JH, Jonides J, Macdonald A 3rd, et al. CNTRICS final task selection: working memory. Schizophr Bull. 2009;35:136–52. https://doi.org/10.1093/schbul/sbn153.
Shalev N, Humphreys G, Demeyere N. Manipulating perceptual parameters in a continuous performance task. Behav Res. Methods. 2018;50:380–91. https://doi.org/10.3758/s13428-017-0877-7.
Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–21. https://doi.org/10.1016/j.tics.2014.04.012.
Wessel JR, Aron AR. It’s not too late: the onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2015;52:472–80. https://doi.org/10.1111/psyp.12374.
Donchin E. Presidential address, 1980. Surprise!… Surprise? Psychophysiology. 1981;18:493–513. https://doi.org/10.1111/j.1469-8986.1981.tb01815.x.
Johnson R Jr. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–84. https://doi.org/10.1111/j.1469-8986.1986.tb00649.x.
Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–32. https://doi.org/10.1037/0033-2909.131.4.510.
Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–48. https://doi.org/10.1016/j.clinph.2007.04.019.
Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35:96–103. https://doi.org/10.1016/0006-3223(94)91198-3.
Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33:48–60. https://doi.org/10.1016/j.neubiorev.2008.08.011.
Swerdlow NR, Bhakta SG, Talledo JA, Franz DM, Hughes EL, Rana BK, et al. Effects of amphetamine on sensorimotor gating and neurocognition in antipsychotic-medicated schizophrenia patients. Neuropsychopharmacology. 2018;43:708–17. https://doi.org/10.1038/npp.2017.285.
Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. https://doi.org/10.1016/j.schres.2004.12.019.
Goldberg TE, Bigelow LB, Weinberger DR, Daniel DG, Kleinman JE. Cognitive and behavioral effects of the coadministration of dextroamphetamine and haloperidol in schizophrenia. Am J Psychiatry. 1991;148:78–84. https://doi.org/10.1176/ajp.148.1.78.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP): Biometrics Research. New York: New York State Psychiatric Institute; 2002.
Chou HH, Talledo JA, Lamb SN, Thompson WK, Swerdlow NR. Amphetamine effects on MATRICS consensus cognitive battery performance in healthy adults. Psychopharmacology. 2013;227:165–76. https://doi.org/10.1007/s00213-012-2948-x.
Wilkinson GS, Robertson GJ. Wide range achievement test—Fourth edition: Professional manual. Lutz, FL: Psychological Assessment Resources; 2006.
Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. https://doi.org/10.1016/j.biopsych.2004.06.023.
Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP. Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology. 2003;28:640–50. https://doi.org/10.1038/sj.npp.1300086.
Talledo JA, Sutherland Owens AN, SchortinghuisT, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology. 2009;204:165–75. https://doi.org/10.1007/s00213-008-1446-7.
Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley & Sons; 1966.
McNicol D. A primer of signal detection theory. London: George Allen & Unwin; 1972.
Frey PW, Colliver JA. Sensitivity and responsivity measures for discrimination learning. Learn Motiv. 1973;4:327–42.
Nolan H, Whelan R, Reilly RB. FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods. 2010;192:152–62. https://doi.org/10.1016/j.jneumeth.2010.07.015.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009.
MacQueen DA, Minassian A, Henry BL, Geyer MA, Young JW, Perry W. Amphetamine modestly improves Conners’ continuous performance test performance in healthy adults. J Int Neuropsychol Soc. 2018b;24:283–93. https://doi.org/10.1017/S135561771700090X.
Ilieva IP, Hook CJ, Farah MJ. Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: a meta-analysis. J Cogn Neurosci. 2015;27:1069–89. https://doi.org/10.1162/jocn_a_00776.
Stige S, Fjell AM, Smith L, Lindgren M, Walhovd KB. The development of visual P3a and P3b. Dev Neuropsychol. 2007;32:563–84. https://doi.org/10.1080/87565640701361096.
Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. https://doi.org/10.3758/cabn.3.1.17.
Greenhouse I, Wessel JR. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology. 2013;50:900–8. https://doi.org/10.1111/psyp.12070.
O’Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15:1729–35. https://doi.org/10.1038/nn.3248.
Philiastides MG, Heekeren HR, Sajda P. Human scalp potentials reflect a mixture of decision-related signals during perceptual choices. J Neurosci. 2014;34:16877–89. https://doi.org/10.1523/JNEUROSCI.3012-14.2014.
Twomey DM, Murphy PR, Kelly SP, O’Connel RG. The classic P300 encodes a build-to-threshold decision variable. Eur J Neurosci. 2015;42:1636–43. https://doi.org/10.1111/ejn.12936.
Acknowledgements
We would like to thank Dr. Mark A. Geyer and Ms. Mahalah R. Buell for their support and Ms. Maria Bongiovanni for assistance with preparation of manuscript.
Funding
The current project was funded by NIMH UH3 MH109168 and R01DA043535.
Author information
Authors and Affiliations
Contributions
SGB: conceptualization, methodology, writing—original draft, investigation, data curation, supervision, project administration, funding acquisition. JFC: conceptualization, methodology, software, formal analysis, writing—original draft, funding acquisition. GAL: conceptualization, methodology, resources, writing—review and editing, funding acquisition. NRS: conceptualization, methodology, writing—review and editing, investigation, data curation, supervision, project administration, funding acquisition. LB, JAT, JEK, BZR, and JAN: investigation, data curation, supervision, project administration. JLB and JWY: conceptualization, methodology, writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
JWY has received pharmaceutical funding from Sunovion Pharmaceuticals, Gilgamesh, and Heptares unrelated to the current work. All other authors report no biomedical financial interests of potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bhakta, S.G., Cavanagh, J.F., Talledo, J.A. et al. EEG reveals that dextroamphetamine improves cognitive control through multiple processes in healthy participants. Neuropsychopharmacol. 47, 1029–1036 (2022). https://doi.org/10.1038/s41386-021-01257-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01257-2