Abstract
How we perceive and interpret signals from others’ behavior, known as social-emotional information processing (SEIP), is key when responding to social threat. Impulsively aggressive individuals, behaviorally, demonstrate impaired SEIP for encoding relevant social stimuli, attribution of intent of the other person in the interaction, and responding negatively to potentially threatening social situations. In this study, we sought to explore how neural processing differs between healthy controls (HC) and individuals with impulsive aggressive behavior (individuals with Intermittent Explosive Disorder, I-IED), during a validated SEIP paradigm. Forty-five adults (19 I-IED, 26 HC) participants underwent a validated SEIP tasks during an fMRI scan. The task utilized video clips depicting a socially ambiguous, but possibly aggressive (AGG), act by one person to another and control video clips in which where possibly aggressive act does not occur (CON). Behavioral anomalies in SEIP are also manifest in altered neural activation in distributed networks/brain regions in each phase of SEIP examined. Overall, neural responses during the SEIP paradigm were characterized as reduced discrimination of the AGG vs. CON videos for I-IEDs compared to HCs. These data suggest the presence of compromised neural circuits underlying impaired social cognition in individuals with IED and highlights potential neural targets of intervention for impaired social cognition in I-IED and other behavioral disorders as well.
Similar content being viewed by others
Introduction
Impulsive aggressive behavior is categorized as Intermittent Explosive Disorder (IED) in the DSM-5 [1] a disorder with past year, and lifetime, prevalence of 2.6% and 4.0%, respectively [2, 3]. Individuals with IED, and those with prominent histories of impulsive aggression, are more likely to manifest evidence of relevant biological alterations, such as a reduction in central serotonin function [4], which suggest reduced inhibitory control [5]. When “triggered”, these individuals respond quickly with negative affect and attack the perceived “threat” [6].
Understanding what triggers an impulsive aggressive response involves several considerations. Perceiving a threat is one, and individuals with IED are likely to perceive threat even when threats are minimal or ambiguous in nature. For example, those with IED are more attuned to anger, and ascribe anger to non-anger related vignettes [7], more likely to attribute hostile intent to others, and endorse anger in other studies where brief vignettes were ambiguous about the motives of the primary “actor” [8,9,10,11]. In addition, neuroimaging studies show that amygdala responses to social threat are greater in those with IED than controls [12, 13]. and that areas in the brain relevant to social processing have lower gray matter volume [14]. If so, it is understandable how the combination of a lowered threshold to respond to threat, coupled with a cortico-limbic system biased to detect social threat signals, even when minimal, could set those with IED to respond more aggressively than others.
How we perceive and interpret signals from others’ behavior is known as social-emotional information processing (SEIP) [15]. SEIP describes processes involved in understanding the nature of how others are behaving towards us and influences how we respond. SEIP is key in situations when responding involves adverse (e.g., aggressive) social threat [16]. Alterations in SEIP for those with IED may underlie more frequent aggression towards others [8,9,10,11].
Before choosing a course of action in response to a social threat, there are three important components to SEIP [15, 17, 18]. These involve “encoding”, “attribution of intent”, and “emotional response”. Encoding refers to receiving social-emotional information and having it available in real time to guide the assessment of behavioral responses in the social interaction. Attribution involves one’s assessment regarding the intentions of the other person in the interaction. For example, was the intention hostile (i.e., this person wants to hurt me) or benign (i.e., the person acted in this way unintentionally)? Emotional response refers to the nature/magnitude of the emotional response to the behavior of the other. Here, emotional response is low when attribution is “benign” and high (angry) when “hostile”. Those with IED have lower encoding, higher hostile attribution, and negative/angry emotional responses compared with non-aggressive controls [10].
To further understand the neural substrates underlying these SEIP components relevant to situations that may lead to aggressive responses, we developed a video-based assessment of SEIP (V-SEIP) suitable for the fMRI environment. The V-SEIP yields similar behavioral results as an established SEIP questionnaire. This V-SEIP contains brief video clips showing adverse but ambiguous (i.e., possibly aggressive) and neutral actions (i.e., control condition). In a study of healthy control participants [19], the aggressive acts yielded greater activation in threat-detection cortex including amygdala and ventrolateral prefrontal cortex during encoding, regions often jointly activated or connected during other threat-processing tasks [12, 13]. During a hostile attribution judgment of the actors, greater mentalization-associated cortex activation occurred for the ambiguous/aggressive videos, including precuneus, inferior parietal lobule, temporal cortex, caudate, thalamus, and cerebellum. Mentalization is the process of inferring another person’s mental state and often includes these regions mentioned, as well ventromedial prefrontal cortex [20]. However, ventromedial prefrontal cortex was not differentially activated by the two video types. Instead, there was greater activation in other aspects of frontal cortex likely associated with memory and other cognitive operations that were more highly engaged for making the judgment about the actor’s intent for the ambiguous/aggressive videos relative to the neutral ones, where the task was easier and less cognitively demanding. Finally, during assessment of expected negative emotional response, aggressive acts elicited greater activation in midbrain associated regions including the periaqueductal gray (PAG) and substantia nigra, associated with emotional stressors and negative emotional expression [21, 22].
In this paper, we compare those with IED to healthy controls (HC) during the V-SEIP task. We hypothesized that for aggressive vs. control videos, IED study participants would: (a) demonstrate greater activation during encoding in the amygdala while demonstrating lower activation in ventrolateral prefrontal cortex, per prior observation of these regions during threat processing in IED [12, 13, 23, 24], (b) demonstrate less activation in regions related to mentalization that the task conditions are sensitive to (e.g., precuneus, temporal and parietal lobes) [20] during attribution, and (c) demonstrate more activation in regions related to threat-related defense during negative emotional response (e.g., PAG) [21].
Methods
Participants
Forty-five right-handed adult participants completed this study. All were medically healthy, medication-free, and were systematically evaluated in regard to aggressive and other behaviors as part of a larger program designed to study the biological and phenomenological correlates of aggressive, and other personality-related, behaviors. Participants were recruited from public service announcements, newspaper and public transportation advertisements, and flyers, with some targeting those with problematic anger or aggression and some targeting HC. All subjects gave written informed consent. This study was approved by the IRB at the University of Chicago. The fMRI data from the healthy control study participants, only, have recently been published [19].
Diagnostic assessment
Syndromal and personality disorder diagnoses were made according to DSM-5 criteria [1]. Diagnoses were made using information from: (a) the Structured Clinical Interview for DSM Diagnoses (SCID) [25] for syndromal disorders and the Structured Interview for the Diagnosis of DSM Personality Disorder [26] for personality disorders, (b) clinical interview by a research psychiatrist, and (c) review of all other available clinical data. The research diagnostic interviews were conducted by individuals with graduate degrees in mental health, and training that yielded good to excellent inter-rater reliabilities (mean kappa of 0.84 ± 0.05; range: 0.79 to 0.93) across mood, anxiety, substance use, impulse control, and personality disorders. Participants with a life history of bipolar disorder, schizophrenia (or other psychotic disorder), or intellectual disability were excluded from this study as were subjects with a current history of alcohol or other substance use disorder. Diagnoses for I-IED participants are displayed in Table 1. For IED participants, most (79%) reported: (a) history of formal psychiatric evaluation and/or treatment (63%), or (b) history of behavioral disturbance during which the subject, or others, thought they should have sought mental health services but did not (16%).
Behavior and trait assessments
Aggression and impulsivity
Aggression was assessed with the Aggression score from the Life history of aggression (LHA) [27] assessment and with the Verbal and Physical Aggression scores from the Buss-Perry Aggression Questionnaire (BPAQ) [28]. LHA and BPAQ scores were highly correlated (r = 0.71, p < 0.001) and, thus, a Composite Aggression score was calculated by taking the average of the z-scores for the LHA and BPAQ. Impulsivity was assessed with the Life History of Impulsive Behavior (LHIB) [29] assessment and with the Barratt Impulsiveness Scale (BIS-11) [30]. LHIB and BIS-11 scores were also correlated (r = 0.58, p < 0.001) and a Composite Impulsivity score was calculated in the same fashion as the Composite Aggression score. In addition, we employed the agreeableness scale score from the NEO Five Factor Inventory [31] as a contrast for aggression and impulsivity scores.
Social cognition
Assessment of hostile and nonhostile social cognition was performed using the Hostile Automatic Thought questionnaire (HAT) [32], a 30-item questionnaire designed to measure hostile automatic thoughts (e.g., “I wish this person would shut up and go away”), and the Positive Automatic Thoughts (PAT) [33], a similar 22-item questionnaire to assess positive automatic thoughts (e.g., “I have a good way with others”).
Neuroimaging
Task design (Fig. S1 in Supplementary Materials)
A scanner-compatible version of the Video Social-Emotional Information Processing (V-SEIP) task [19] was used. It consisted of 40 trials presented over four runs in one scan session. The videos were 10–15 s in length. A trial consisted of three phases: Encoding (ENC), Hostile Attribution (HA), and Negative Emotional Response (NER), each separated by a variable period of fixation on a central crosshair (4–12 s, jittered). The ENC phase was the last 2–8 s of each video clip depicting a situation between two individuals, portrayed by professional actors. There were ten unique situations. Each was shown at some point during the scan session with an adverse/aggressive, but of ambiguous intent, or a neutral ending (AGG or CON, respectively). Also, each of the ten unique situations was filmed with a male actor as the primary subject, and a second time with a female primary subject. Hence, there were 20 pairs of visually identical videos, differing only in the final seconds, which were either AGG or CON, but of the same length. The time point at which Actor A experiences the adverse/aggressive action was used as the start of the ENC phase. The corresponding CON ending was coded temporally similarly with its paired AGG version, so AGG and CON videos were matched for onset and duration. The HA and NER phases of a trial were presented in pseudo-random order after each video. In the HA phase, a written question appears that reads “How likely is it that Actor B intended to hurt or embarrass Actor A in the video?” The bottom of the screen shows a 1–4 scale (1 = “not at all” and 4 = “very”) and subjects press a corresponding button on a 4-button box with their right hand. Following a variable fixation period (4–8 s), the emotional response phase begins with a written question that reads: “How angry or upset would you be if this happened to you?” on the same 4-point scale. Each written question was presented for 4 s. Each of the 40 videos with HA and NER response phases (10 situations × 2 endings × 2 sexes of primary subject) was a trial. Trial order was pseudo-randomized and presented in the same order for all participants, with five AGG and five CON trials per run, yielding a total of four runs.
fMRI data acquisition
Scans were conducted on a Philips Achieva Quasar 3T MRI Scanner. For co-registration to the functional data, a high-resolution T1-weighted image was acquired (TR = 8.0 ms, TE = 3.5 ms, Flip Angle = 8°, FOV = 240 mm, slice thickness/gap = 1.0/0 mm). fMRI data was collected using dynamic T2*-weighted gradient echo planar imaging with BOLD (blood oxygenation level dependent) contrast (TE = 25 ms, TR = 2000 ms, flip angle = 77°, FOV = 192 mm, 30 4 mm oblique axial slices approximately parallel to the AC-PC line, 0.5 mm slice gap). A modified, high efficiency, z-shim compensation was applied to the four slices covering the orbitofrontal cortex to minimize susceptibility artifacts [34].
fMRI preprocessing
fMRI data were preprocessed in SPM12 (Wellcome Department of Cognitive Neurology, London) following the steps described in Coccaro et al. [19]. Briefly, steps included slice time correction and realignment. Structural images were co-registered to the mean functional image and used to normalize functional and structural data to MNI space. Functional images were filtered at 128 Hz high pass filter and smoothed with a Gaussian kernel of 6 mm.
Statistical analyses
For fMRI data, first-level models of activation to each task condition were specified and estimated using a general linear model per voxel per subject using the preprocessed time series. ENC, HA, and NER phases were each modeled for both AGG and CON trials by using box-car functions convolved with the canonical hemodynamic response function. Motion parameters were included as regressors of no interest. Button presses for both response phases were also used as regressors of no interest, so that the full 4 s period of viewing the questions was evaluated for brain activity inclusive of that associated with considering and answering the question, but not with the motor aspects of responding that might have varied in terms of onset across trials. For each subject, statistical parametric maps, e.g., contrast maps, were created for each task phase contrasting the two different trial types (AGG > CON) for ENC, HA, and NER. These three maps were the three dependent variables on which the groups were compared. Each map represented the activation during aggressive videos relative to neutral videos. Each subject’s contrast map was the input for 2-sample t tests to compare the two groups, with age, race, and sex as covariates. These second-level analyses were evaluated at a whole-brain level (excluding high-probability white matter and CSF voxels) and corrected for multiple comparisons (p < 0.008, FWE). Post hoc pairwise tests were performed to assist interpretation of group or task condition differences. For behavioral data collected during and outside of the scanning sessions, responses were analyzed separately for HA and NER questions using a paired samples t test. Lastly, we assessed correlation of any group differences in activation with the aggression and other behavioral measures.
Results
Participants
Table 2 displays the demographic and psychometric characteristics of the two groups. Demographically, the groups only differed in ethnic composition where the IED group had a smaller proportion of European-American participants compared with the HC group (11% vs. 38%). Psychometrically, the groups differed as expected and IED participants had significantly higher scores for the aggression, anger, impulsivity, and hostile automatic thought variables, and lower scores on agreeableness and on positive automatic thought variables, compared with HC participants.
V-SEIP behavioral data during scanning
While V-SEIP ENC scores could not be obtained during scanning, we did obtain HA and NER scores during the scanning session (Table S1 in Supplementary Materials). V-SEIP HA scores in the scanner were significantly greater for I-IED vs. HC (F [1,41] = 17.21, p < 0.001), AGG vs. CON Video (F [1,41] = 242.56, p < 0.001). The same was true for NER scores for I-IED vs. HC (F [1,41] = 20.01, p < 0.001), AGG vs. CON Video (F [1,41] = 339.13, p < 0.001).
fMRI V-SEIP data (Tables 3 and S3)
Encoding phase
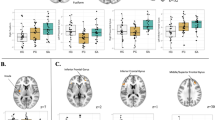
For the between group comparison of AGG > CON, IED participants had decreased activation in a cluster comprised of medial orbitofrontal cortex and anterior cingulate (Fig. 1a). Post hoc testing showed that this was characterized by I-IED having a stronger negative BOLD response to the AGG video relative to CON, whereas HCs did not show a strongly differential activation pattern, though also had negative BOLD activation to both conditions (Fig. 2a).
Locations of significant differences for each V-SEIP Phase between IED and HC study participants (a for encoding; (b) for hostile attribution, (c) for negative emotional response). Data is depicted in binarized fashion, with a single color (red) indicating voxels that survived the statistical threshold (p < 0.008, FWE).
Hostile attribution phase
For the between group comparison of AGG > CON, I-IEDs had reduced activation in superior, middle, and inferior frontal gyri corresponding to left ventrolateral prefrontal cortex (BA 44, 45, 46), and to bilateral frontal and prefrontal cortex corresponding to Brodmann areas 6, 9, and 10 (Fig. 1b). Reductions were also noted in bilateral inferior parietal lobule, precuneus, middle temporal gyri, and cerebellar hemispheres. Across these clusters, both groups had mean positive BOLD responses to all task conditions. However, HC had stronger activation for AGG than CON conditions, while I-IEDs did not show differential activation between the conditions (Fig. 2b).
Negative emotional response phase
For the between group comparison of AGG > CON, I-IED participants had reduced activation in bilateral periaqueductal gray (PAG; Fig. 1c). The PAG differences were characterized by HCs showing stronger activation to AGG than CON videos, and I-IEDs showing no differential strength to their positive BOLD responses (Fig. 2c).
Associations between behavior/traits and activation
Extracted mean activation (beta weights) for each subject in each significant cluster (clusters were converted to mask files which were then applied to subjects’ contrast map files to obtain means using AFNI’s 3dROIStats) for each V-SEIP phase were highly correlated [p < 0.001 for all correlations], justifying the data-reduction step of creating a composite mean activation variable (mean of the AGG > CON values) for each subject and task phase to assess in relation to behavior and trait measures across all subjects. Composite activations correlated significantly, and inversely, with Composite Aggression (but not impulsivity) and Hostile Automatic Thought in each V-SEIP phase (Table S2 in Supplementary Materials). Corresponding correlations with NEO Agreeableness and Positive Automatic Thought scores were smaller in magnitude and, as expected, in the opposite direction.
Discussion
These data suggest that anomalies in SEIP observed in impulsively aggressive individuals are manifest in altered neural activation in distributed networks/brain regions in each phase of SEIP investigated: encoding, hostile attribution, and negative emotional response. Neural alterations were characterized as either reduced or sharpened discrimination of the AGG vs. CON videos for I-IEDs compared to HCs (see Fig. S5 for summary of predictions and findings).
For encoding, IEDs had lower activation than HCs but also displayed stronger neural discrimination than HCs in a cluster located in the medial orbitofrontal cortex and the anterior cingulate. This neural activity occurred while viewing the time where one person acted adversely/aggressively toward another person relative to viewing a visually similar non-aggressive action. I-IEDs had a stronger negative BOLD response than HCs to the aggressive action. Negative BOLD responses, shown in this region for all task conditions and groups, are more difficult to interpret than positive BOLD responses [35]. However, a parallel observation has been made in PET imaging studies of healthy individuals imagining aggressive acts, showing reduced cerebral blood flow in medial orbitofrontal cortex relative to neutral scenes [36]. This supports an interpretation of lowered neural activity in this brain area in association with processing aggressive actions. Further, Beyer et al. [37] reported that healthy people had lower (e.g., more negative) fMRI BOLD activation in medial orbitofrontal cortex in association with responding with more aggression to a perceived aggressor. Taken together these studies support a model in which suppressed medial OFC activation is associated with aggressive behavior in I-IED. To further understand the stronger negative activation in medial OFC in I-IEDs compared with HCs, composite aggression scores were most strongly associated with deactivation in virtually all areas in each V-SEIP phase (Supplementary Figs. S2–S4).
Notably, we did not find differences in amygdala activation for I-IEDs relative to HC in this phase, as predicted. Our prior analysis of the HCs in this sample indicated that, compared to the control videos, the aggressive videos activated bilateral amygdala during this encoding phase [19]. The absence of an I-IED vs. HC difference in amygdala response in this study may be due to differences in method and nature of the stimuli. While presenting stimuli of clear social threat with “emotional faces” elicits an I-IED-Control difference in the amygdala [12, 13], such may not be the case for the V-SEIP stimuli, which are socially ambiguous in nature.
In the hostile attribution phase, I-IEDs had little differentiation in activation for aggressive vs. control videos across several brain regions relative to HCs. As reported previously [19], an extensive degree of activation was needed for HCs to assess the intent of the adverse action, likely due to their ambiguous nature, generating high mental effort. The reduction in this neural response for I-IEDs suggests the possibility that making a judgment on attribution of intent was not as engaging in I-IEDs as it was for HCs. In other words, the known hostile attribution bias of IED may have made it easier for I-IEDs to determine a rating of hostile intent because the actions seemed less ambiguous (and instead more hostile) to them. Indeed, I-IEDs rated the actors as having more hostile intent, and lower differences between aggressive and control video-related activation during this phase of the task was associated with these higher hostile attribution ratings. The lower activation in these varied regions more specifically reflects lower engagement of functions such as memory retrieval, working memory (prefrontal cortices) and metallization (precuneus/posterior temporal gyrus). While it cannot be directly known from this data, it seems unlikely I-IEDs have widespread changes to these complex cognitive systems that lead to greater hostile attribution, as no such prior observation of widespread cognitive deficits or neural system alterations have been made. Rather, the deactivation of medial orbitofrontal cortex at the onset of the adverse event (encoding phase) may set up a bias to perceive hostility given concurrent disinhibited neural systems for aggressive behavioral responding. That said, these explanations remain to be directly tested.
Reductions in activations during the NER phase in the PAG further characterizes the functional compromise in neural systems of I-IEDs in response to social threat. For this phase, the alteration is in association with considering one’s own presumed negative emotions if they experienced the adverse or neutral action. For PAG, the pattern of group differences was similar to that of most of the differences for the HA phases. Activation was positive and HCs showed stronger activation for aggressive versus control videos while I-IEDs displayed no differential activation between conditions. The PAG is known to activate in response to negative emotional stressors and to control emotional expression [38]. Known as a pivotal component of a central “survival network”, [22] PAG is a behaviorally important source of descending control that is activated in response to a variety of emotional and environmental stressors, such as fear, anxiety, and pain [21], and is crucial in controlling the expression and co-ordination of responses in these contexts [39,40,41]. HCs displayed the expected differential response in PAG for more adverse emotional experiences. However, even though I-IEDs fail to show this differential response, they still estimated a stronger negative response in accordance with their greater hostile attribution ratings. This may reflect a key alteration in IED whereby their negative emotional responses may not derive as much from mechanisms mediated in PAG and is in the opposite direction of alteration predicted. No other significant cluster distinguished HCs from IEDs to offer clues. These observations require further study.
We also observed correlations with assessments of aggression and related variables. For example, we observed inverse correlations between contrast activation measures for each SEIP phase and composite aggression scores. These relationships were not due to having participants with high (I-IED) and low (HC) aggression scores as the finding was with all subjects in the analysis, and there is overlap on aggressive scores between the groups (Supplementary Figures). Similar inverse correlations were observed with Hostile Automatic Thought scores reflecting a direct relationship between aggression and hostile thoughts. As expected, we observed positive correlations between the composite contrast activations and NEO “agreeableness” and Positive Automatic Thought (PAT) scores.
Strengths of this study include the fact that we have shown that the V-SEIP paradigm, as a behavioral task, is valid and reliable as an assessment of hostile social cognition. Additional strengths are the fact that study participants were well characterized diagnostically and comprehensively assessed regarding aggression and social cognitive variables. Limitations include a sample of modest size. In addition, the fMRI parameters of the task design may not allow for a truly independent comparison of fMRI BOLD responses across different task phases. A slower, event-related, design allowing for a longer wash-out of previously elicited fMRI BOLD responses may need to be tested in future studies. Repetition effects, in which study participants viewed similar clips up to four times, may have rendered our stimuli less novel during the second half of the task. That said, no differences were observed in fMRI BOLD responses among the three phases between the first and second half of the task [19]. We note that the V-SEIP stimuli are not first-person in nature and, thus, behavioral and/or fMRI BOLD responses might not be the same as if stimuli were directed at the study participant. However, a similar study using first-person stimuli with unambiguously aggressive videos clips found similar results to ours [42].
In conclusion, these data suggest the presence of compromised neural circuits underlying social cognition in individuals with IED. While replication in larger samples, and perhaps with modified task parameters, are warranted, it is possible that this study has highlighted potential neural targets of intervention for social cognition not only in those with IED but others as well.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, D. C.: American Psychiatric Association Press; 2013.
Coccaro EF, Lee RJ. Disordered aggression and violence in the United States. J Clin Psychiatry. 2020;81:19m12937.
Coccaro EF. Psychiatric comorbidity in intermittent explosive disorder. J Psychiatr Res. 2019;118:38–43.
Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169:577–88.
Krakowski M. Violence and serotonin: influence of impulse control, affect regulation, and social functioning. J Neuropsychiatry Clin Neurosci. 2003;15:294–305.
Lee RJ, Coccaro EF. Neurotransmitters and Intermittent Explosive Disorder. In: Coccaro EF and McCloskey MS, editors. Intermittent explosive disorder: etiology, assessment, and treatment. London, UK: Academic Press; 2019. p. 87–110.
Patoilo MS, Berman ME, Coccaro EF. Emotion attribution in intermittent explosive disorder. Compr Psychiatry. 2021;106:152229.
Coccaro EF, Noblett KL, McCloskey MS. Attributional and emotional responses to socially ambiguous cues: validation of a new assessment of social/emotional information processing in healthy adults and impulsive aggressive patients. J Psychiatr Res. 2009;43:915–25.
Coccaro EF, Fanning J, Lee R. Development of a Social Emotional Information Processing Assessment for Adults (SEIP-Q). Aggressive Behav. 2017;43:47–59.
Coccaro EF, Fanning JR, Fisher E, Couture L, Lee RJ. Social emotional information processing in adults: Development and psychometrics of a computerized video assessment in healthy controls and aggressive individuals. Psychiatry Res. 2017;248:40–7.
Coccaro EF, Fanning JR, Keedy SK, Lee RJ. Social cognition in Intermittent Explosive Disorder and aggression. J Psychiatr Res. 2016;83:140–50.
Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–78.
McCloskey MS, Phan KL, Angstadt A, Fettich KC, Keedy S, Coccaro EF. Amygdala hyperactivation to angry faces in intermittent explosive disorder. J Psychiatr Res. 2016;79:34–41.
Coccaro EF, Fitzgerald DA, Lee R, McCloskey M, Phan KL. Frontolimbic morphometric abnormalities in intermittent explosive disorder and aggression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:32–8.
Crick NR, Dodge KA. Social information-processing mechanisms in reactive and proactive aggression. Child Dev. 1996;67:993–1002.
Dodge KA, Somberg DR. Hostile attributional biases among aggressive boys are exacerbated under conditions of threats to the self. Child Dev. 1987;58:213–24.
Huesmann LR. An information processing model for the development of aggression. Aggressive Behav. 1988;14:13–24.
Dodge KA. Translational science in action: hostile attributional style and the development of aggressive behavior problems. Dev Psychopathol. 2006;18:791–814.
Coccaro EF, Keedy S, Lee R, Phan KL. Neuronal responses to adverse social threat in healthy human subjects. J Psychiatr Res. 2021;136:47–53.
Atique B, Erb M, Gharabaghi A, Grodd W, Anders S. Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage. 2011;55:1899–911.
Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305.
Watson TC, Koutsikou S, Cerminara NL, Flavell CR, Crook JJ, Lumb MB, et al. The olivo-cerebellar system and its relationship to survival circuits. Front Neural Circuits. 2013;7:72.
Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984;230:465–96.
Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID). New York: Psychiatric Institute, Biometrics Research; 1997.
Pfohl B, Blum N, Zimmerman M, University of Iowa. Dept. of Psychiatry. Structured interview for DSM personality disorder: SIDP. Washington D.C.: American Psychiatric Press; 1997.
Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–57.
Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–9.
Coccaro EF, Schmidt-Kaplan CA. Life history of impulsive behavior: development and validation of a new questionnaire. J Psychiatr Res. 2011;46:346–52.
Patton J, Stanford M, Barratt E. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74.
McCrae RR, Costa PT. A contemplated revision of the NEO five-factor inventory. Personal Individ Differ. 2004;36:587–96.
Snyder CR, Crowson JJ, Houston BK, Kurylo M, Poirier J. Assessing hostile automatic thoughts: development and validation of the HAT scale. Cogn Ther Res. 1997;21:477–92.
Ingram RE, Kendall KC, Siegle G, Guarino JSC, McLaughlin SC. Psychometric properties of the positive automatic thoughts questionnaire. Psychol Assess. 1995;7:495–507.
Du YP, Dalwani M, Wylie K, Claus E. Reducing susceptibility artifacts in fMRI using volume-selective z-shim compensation. Magn Reson Med. 2007;57:396–404.
Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG–BOLD–CBF study in humans. NeuroImage. 2014;94:263–74.
Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry. 2000;157:1772–81.
Beyer F, Münte TF, Göttlich M, Krämer UM. Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb Cortex. 2015;25:3057–63.
Jürgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979;167:367–78.
Carrive P, Leung P, Harris J. Conditioned fear to context is associated with increased Fos expression in the caudal ventrolateral region of the midbrain periaqueductal gray. Neuroscience. 1997;78:165–77.
Fanselow MS, Kim JJ, Young SL, Calcagnetti DJ, Decola JP, Helmstetter FJ. Differential effects of selective opioid peptide antagonists on the acquisition of pavlovian fear conditioning. Peptides. 1991;12:1033–7.
Walker P, Carrive P. Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience. 2003;116:897–912.
Fehr T, Achtziger A, Roth G, Struber D. Neural correlates of the empathic perceptual processing of realistic social interaction scenarios displayed from a first-order perspective. Brain Res. 2014;1538:141–58.
Funding
This work was supported by grants from the NIMH (R21-MH99393) and the Pritzker-Pucker Family Foundation to EFC. In addition, this work was completed in part with resources provided by the University of Chicago’s Research Computing Center and by MRI Research Center.
Author information
Authors and Affiliations
Contributions
Drafting the work: EFC, SK. Revising it critically for important intellectual content: MM, RL, and KLP. Final approval of the version to be published: EFC, SK, MM, RL, and KLP. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: EFC, SK, MM, RL, and KLP.
Corresponding author
Ethics declarations
Competing interests
EFC reports being on the Scientific Advisory Board of Azevan Pharmaceuticals, Inc. and reports that he is a consultant to Avanir Pharmaceuticals, Inc. SK, RL, KLP, and MM have nothing to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Coccaro, E.F., Keedy, S., Malina, M. et al. Neuronal responses in social-emotional information processing in impulsive aggressive individuals. Neuropsychopharmacol. 47, 1249–1255 (2022). https://doi.org/10.1038/s41386-022-01296-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01296-3