Abstract
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published in 2013, includes an alternative model of personality disorders (AMPD) focusing on a maladaptive trait model utilized to diagnose several personality disorders. Borderline personality disorder (BPD) and antisocial personality disorder (ASPD) are two conditions categorized by AMPD that exhibit high rates of violence and aggression. Several of the traits outlined in the AMPD, including hostility, impulsivity, risk-taking, and callousness, have been previously linked to aggression in BPD and ASPD. However, to the best of our knowledge, there has never been a synthesis of neuroimaging studies that have investigated links between these traits and aggression in BPD and ASPD. To overcome this gap, we conducted a systematic review under the PRISMA framework to locate neuroimaging articles published since the release of AMPD linking trait anger/hostility, impulsivity, risk-taking, and callousness to aggression in BPD and ASPD. Key findings included the following: i) anger/hostility, associated with alterations in the interplay between prefrontal and subcortical regions (primarily the amygdala), may be a common factor explaining aggressive reactions to response to interpersonal threat or provocation; ii) alterations of fronto-temporal-limbic regions and serotonergic and endocannabinoid signaling systems may link impulsivity to aggression in BPD and ASPD; iii) weaker cortico-striatal connectivity could relate to greater risk taking and greater proclivity for violence. Insufficient evidence from neuroimaging articles was discerned to describe a relationship between callousness and aggression. Overall, results of this review reveal a relative paucity of neuroimaging studies examining AMPD traits relevant to aggression in BPD and ASPD. In addition to encouraging further investigation of neuroimaging markers of AMPD traits linked to aggression, we recommend multi-methodological designs, including the incorporation of other biomarkers, such as hormones and indices of physiological arousal, to fully expand our understanding of aggression in BPD and ASPD.
Similar content being viewed by others
Introduction
Aggression is a common clinical feature of antisocial personality disorder (ASPD; [1]) and borderline personality disorder (BPD; [2]). It contributes to detrimental outcomes in both conditions, with much higher rates of violent offending in both ASPD and BPD [1, 3, 4]. Comorbidity of these conditions is common [5, 6] and associated with increased rates of aggression and violence [3, 7, 8]. Evidence for treatment options targeted at reducing aggression in ASPD [9, 10] and BPD [11] is poor, and development of effective treatments is hampered by a limited understanding of the mechanistic basis of aggression in these conditions. One pathway to a better understanding of such mechanisms is the use of neuroimaging, which can detect structural, functional, and neurochemical abnormalities and link these to subtypes of aggressive behavior [12, 13], thereby identifying potential therapeutic targets.
Most of the initial neuroimaging research in BPD and ASPD failed to consider the potential link between specific personality disorder traits and neural markers of aggression. However, early in the last decade, a shift towards a transdiagnostic, trait-based approach was reflected by the inclusion of an alternative model of personality disorders (AMPD) in Section III of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). This demonstrated that while the field was not yet prepared to abandon categorical diagnoses, a framework for researching the trait-based nature of these disorders had emerged (see Box 1). To advance these approaches, neuroimaging research would optimally link behavioral outcomes, such as aggression, to core traits that overlap across two or more personality disorders. Since the publication of DSM-5 in 2013, there have been a number of reviews examining the neurobiological correlates of aggression in BPD [14,15,16,17], ASPD [18, 19], and personality disorders as a group [20], as well as in non-clinical samples [21]. However, the degree to which DSM-5 has influenced empirical approaches remains unclear. Importantly, no study has systematically appraised the neuroimaging literature linking specific maladaptive personality traits to neural metrics of aggression within these important clinical disorders.
We, therefore, sought to systematically review the literature since 2013, using a trait-based approach. As previous evidence suggests that anger/hostility [22,23,24,25,26], impulsivity [23, 25,26,27], risk taking [22], and callousness/lack of empathy [22, 28], are linked to aggression in BPD and/or ASPD, we focused on studies linking measures of one or more of these four traits to metrics of aggression in BPD and/or ASPD. As ASPD is present in up to 80% of prison samples [29], we also considered studies in samples of incarcerated offenders, in which the Psychopathy Checklist-Revised [29] is commonly used as a measure of the degree of antisociality. As there is significant overlap between these conditions and intermittent explosive disorder (IED), we discuss this condition separately (see Box 2).
Methods
A systematic literature search was undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guide. The search was restricted to papers published between 2013 and 2022 and the following databases were searched in October 2022: MEDLINE® ALL, Embase (via Ovid), APA PsycInfo®, EBSCO CINAHL, and APA PsycArticles® (via ProQuest). We searched for papers from 2013 onwards, as this was the date when DSM-5 was published and the AMPD was first introduced.
The search was conducted by an information specialist (NT, see Acknowledgements), using a combination of free text terms (searching the title and abstract) and relevant controlled vocabulary headings customized for each database, as well as advanced search syntax (truncation, Boolean logic AND/OR, and proximity searching), to ensure all relevant studies were identified. The search terms included the following themes, with synonyms to describe each: borderline personality disorder or emotionally unstable personality disorder or antisocial personality disorder or psychopathy, traits (hostility, impulsivity, risk- taking or callousness), aggression, and neuroimaging.
Studies were initially included if they were (1) published as a peer-reviewed article with original data in adult samples using structural MRI (sMRI), functional MRI (fMRI, including measures of functional connectivity), positron emission tomography (PET), magnetic resonance spectroscopy (MRS), and diffusion tensor imaging (DTI); (2) included individuals with BPD and/or ASPD (with or without psychopathy), defined using standardized classification tools (DSM or ICD criteria for BPD and ASPD) or included incarcerated offenders with a Psychopathy Checklist- Revised (PCL-R) score for psychopathy; (3) included a quantifiable, standardized metric of at least one of the following: risk-taking, impulsivity, hostility, or callousness/lack of empathy; and (4) investigated the link between at least one of these traits and a metric of aggression using a neuroimaging technique. Exclusion criteria were the following: 1) review articles; 2) dissertations; 3) letters to the editor; 4) opinion articles; 5) editorials; and 6) case reports or case series.
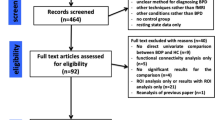
Our PRISMA diagram (see Fig. 1) illustrates our search strategy. Our initial search strategy identified 1,212 records. We then supplemented the search by manual and bibliographic cross referencing and by examining previous systematic reviews and meta-analyses to identify potentially missed studies. This strategy revealed 7 additional records. Once duplicates had been removed, we had 723 records. We screened out 641 records based on titles and abstracts. We retrieved the remaining 82 studies to assess eligibility. We included 17 studies based on the search strategy. The search screening and data extraction were completed independently by three separate researchers: NJK, JT, and KB. Disagreements were discussed and finalized by consensus vote. For each extracted article, we recorded the study author, trait being examined, neuroimaging technique, metric of aggression, sex composition of the sample, personality disorder diagnosis, and main neuroimaging findings. See Table 2.
Results
Neuroimaging studies investigating anger/hostility and aggression
Several studies have examined the relationship between neural correlates of anger/hostility and aggression. We identified five studies including individuals with BPD and four studies with violent offenders with at least the majority having a personality disorder diagnosis – mostly ASPD.
In one study [30], 48 female patients with BPD and 28 healthy women participated in the Social Taylor Aggression Paradigm, a fMRI-compatible modification of the Taylor Aggression Paradigm. As in previous studies [31, 32], healthy women responded with higher activation in the amygdala and orbitofrontal cortex (OFC), as well as with higher aggression in trials with an angry versus neutral looking opponent. However, women with BPD did not show this emotion-dependent modulation. In both groups, there was a positive correlation between amygdala responsivity and aggression; however, in healthy women, this was for angry faces, while in women with BPD, it was for neutral faces. This suggests that, when in a context of provocation, women with BPD might not be able to adequately differentiate neutral/friendly from angry/hostile interpersonal signals, and this biased processing is even higher in those patients who react aggressively.
Another study [33] measured neural correlates of acting out in anger (a proxy marker of aggression) in 15 anger-prone men with BPD and 25 healthy men using an fMRI-compatible emotional approach avoidance task. Similar to a previous study with female BPD patients [34], men with BPD showed a tendency to approach rather than avoid angry faces and reduced ventrolateral prefrontal cortex (vlPFC) activations in incongruent trials, compared to healthy controls. In addition, the tendency to act out in anger (subscale of the State-Trait-Anger-Inventory; STAXI) was negatively related to vlPFC and dorsolateral prefrontal cortex (dlPFC) activation but positively related with amygdala activity in men with BPD. Since similar findings were previously reported in men with ASPD/psychopathy [35], such a deficit in fast emotional action control could be a common neural mechanism of anger-proneness and hostility predisposing an individual to aggressive reactions. A role for the vlPFC is supported by a further study [36], which demonstrated reduced gray matter volume in vlPFC in BPD patients with versus without a history of childhood abuse (the total sample included 18 individuals with BPD and 19 healthy controls). Intriguingly, gray matter in this region was related to both aggression and a form of hostility (“negativism,” as defined by the Buss-Durkee Hostility Inventory) in patients with childhood abuse. Although no sub-analysis of the link between aggression and hostility was performed in this sample, these findings suggest a possible interaction between structural vlPFC atypicality, trait hostility, and aggressive acts in men with BPD.
A further study [37] used a script-driven imagery paradigm to induce feelings of anger with standardized vignettes describing prototypical situations of interpersonal rejection, provocation, and frustration followed by aggressive reactions. Notably, this study included 23 male and 33 female anger-prone patients with BPD as well as 26 healthy men and 30 healthy women, allowing for analysis of sex by group interactions. Findings demonstrated that angerinduction led to increased amygdala activity in only men with BPD and not in healthy men and women with BPD. Male patients with BPD also showed elevated activations in the amygdala, OFC and dlPFC, while imagining anger-induced aggression. Furthermore, trait anger (STAXI subscale) was negatively associated with amygdala-dlPFC connectivity, while trait aggression (Buss–Perry Aggression Questionnaire; BPAQ) correlated positively with the amygdala-thalamus coupling in male patients.
Using fMRI, another group compared a sample of 25 healthy controls with 33 aggressive BPD patients (20 receiving specific anti-aggression group psychotherapy and 13 patients taking part in a non-specific control psychotherapy) [38]. Findings demonstrated a significant reduction in amygdala response and increased dorsomedial prefrontal cortex (dmPFC)-amygdala connectivity to emotional faces (including angry and fearful expressions) in an emotional face matching task in the aggressive patient group. Importantly, changes in amygdala activity and dmPFC-amygdala connectivity were related to changes in aggressive behavior (assessed with the Overt Aggression Scale-modified (OAS-M)) from pre- to post-treatment in the anti-aggression psychotherapy group only. These findings suggest the utility of targeted psychotherapeutic treatment approaches for subgroups of patients with BPD, based on level of aggression, which is in keeping with development of personalized medicine approaches.
Two studies, using an overlapping sample of violent male offenders, provided further insight into the link between neural correlates of anger/hostility and aggression in ASPD. In one [39], a correlation was demonstrated between a composite aggression measure and elevated amygdala reactivity to fearful faces in a similar emotional face matching task that was also reported in 19 incarcerated violent male offenders with personality disorders (mostly ASPD) compared to 28 healthy men. In the other [40], increased responsivity to provocation in the amygdala and striatum and a reduced connectivity between the ventromedial prefrontal cortex (vmPFC) and amygdala as well as striatum was found in 18 offenders, compared to 26 controls. Notably, vmPFC reactivity to provocation was positively related to trait anger (STAXI subscale) and aggression (BPAQ) across groups. In another study [41], violent offenders – the majority with a personality disorder diagnosis, mostly ASPD – were presented auto-taped thoughts and beliefs in response to angry, neutral, and happy situations and asked to either focus on their emotional feelings (engagement condition) or to regulate their feelings (distraction condition). Before and after this task, resting-state fMRI was performed. During anger engagement, increased vlPFC activation was found in 16 violent offenders compared to 18 non-offender controls, while the opposite (increased activity in offenders versus controls) was found in vlPFC and dlPFC during anger distraction [41]. Furthermore, reduced amygdala and vlPFC activity during anger distraction were positively related to self-reported aggression (BPAQ and Reactive Proactive Questionnaire). Analyzing resting state activity patterns in the same sample, a further study [42] found a positive correlation between anger (Anger-Single Target Implicit Association Test) and medial prefrontal cortex (mPFC) activity before the anger task, which increased during the task. Furthermore, the connectivity between mPFC and amygdala decreased in 18 violent offenders, while it increased in 18 controls during the task, and the opposite pattern was found for the connectivity between amygdala and (para)limbic regions.
Together, these studies suggest altered processing of anger-inducing situations in aggressive individuals. This seems to involve both more automatic, limbic reactions as well as prefrontal processes of cognitive control. However, the interplay of these regions, dynamic patterns, and the precise situational triggers remain unclear.
Neuroimaging studies investigating impulsivity and aggression
Several studies of BPD and ASPD have examined the neural correlates of impulsivity and the relationship between impulsivity and aggression using imaging techniques. In general, neuroimaging investigations in BPD have reported structural, metabolic, and functional alterations of fronto-limbic networks that provides a neural basis for emotional dysregulation and impulsive and aggressive behavior [43,44,45]. One study of 31 female BPD participants and 25 female control subjects participated in an fMRI Go/No-Go task that presented negative (e.g., angry, sad, fearful), positive (e.g., happy), and neutral Ekman faces to elicit functional responding [46]. Trait impulsivity in BPD, measured using the Barratt Impulsiveness Scale-11 (BIS-11), was found to positively correlate with activation in the dorsal anterior cingulate cortex (dACC), OFC, dlPFC, and basal ganglia, while aggression negatively correlated with OFC, hippocampus, and basal ganglia activation. Negative emotional context and trait impulsivity, but not aggression, decreased task performance across groups. These results suggest that as an alternative to the “top-down, bottom-up” model proposed for affective interference with cognitive function in women with BPD [47, 48], negative emotion arising from situational stressors interrelates with the pre-existing neurobiology of personality traits, such as impulsivity, resulting in affective interference of neural processing of cognitive functions [46].
A different analysis of the same sample of 51 mixed sex BPD participants compared impulsivity (BIS-11), aggression (LHA), and brain structure using sMRI in high and low lethality suicide attempters [49]. No significant difference was noted between high and low lethality suicide attempters in terms of aggression or impulsivity. However, higher degrees of medical lethality were associated with decreased gray matter volumes across fronto-temporal-limbic regions, and effects of impulsivity and aggression on gray matter volumes differentiated high from low lethality attempters and differed within lethality groups. These results imply that lethality of suicide attempts in BPD could be related to mediation of aggression and impulsivity by specific neural networks.
The same group [50] used [18F]altanserin PET to quantify whether sex had a significant effect on the associations between 5-HT2A binding; personality traits, such as impulsivity and aggression; and suicidal behavior in BPD. Thirty-three BPD patients (mixed sex) and 27 healthy controls (mixed sex), all unmedicated, were examined. The group had previously found effects of sex among healthy volunteers on the association between 5-HT2A binding and aggression [51]. In the current study, among female BPD subjects, trait impulsiveness was inversely related to [18F]altanserin binding potential (BPND) in medial frontal cortex, while aggression was negatively related to BPND in medial OFC. There were no significant relationships between these traits and BPND in male BPD subjects. Additionally, among BPD subjects, aggression, cluster B comorbidity, ASPD, and childhood abuse each related to altanserin binding. Therefore, region-specific differences in 5-HT2A binding related to diagnosis, sex, and history of childhood abuse may relate to the clinical expression of aggression and impulsivity in BPD.
One resting-state investigation explored whether neurochemical systems, including the noradrenergic, dopaminergic, and serotonergic neurotransmitter systems, may be involved in the impulsivity of BPD [52]. This study evaluated the functional connectivity of the main monoamine-producing nuclei within the midbrain and brainstem in 33 unmedicated female participants with BPD and 33 matched healthy controls to relate any altered functioning of these nuclei to the patient’s impulsivity. Although multiple regression did not detect any significant association between impulsivity and altered functional connectivities in the BPD group, BPD patients exhibited stronger functional connectivity from the noradrenergic locus (e.g., locus coeruleus) to the ACC, which was positively correlated with the degree of motor impulsiveness in the BPD sample. Furthermore, while controlling for aggression, stronger functional connectivity was detected between the serotonergic nucleus centralis superior (NCS) and the frontopolar cortex in patients versus controls. While the fMRI modality utilized in the current study cannot directly implicate dysfunction of monoamine neurotransmission in BPD, enhanced locus coeruleus-ACC resting state functional connectivity in women with BPD and its link to motor impulsiveness could indicate noradrenergic dysfunction in neural inhibitory control networks, while increased NCS-frontal pole resting state functional connectivity could implicate serotonergic signaling in prefrontal control of aggressive behavior.
An investigation that sampled 26 females with BPD, 22 females with attention deficit hyperactivity disorder (ADHD), and 30 female healthy controls also considered neurochemical underpinnings. This study explored the relationship between measures of impulsivity and aggression and ACC glutamate to total creatine ratios (Glu/tCr) and GABA levels using single voxel 1H magnetic resonance spectroscopy [53]. Self-rating scales, including the BIS-11 and Brown Goodwin Lifetime History of Aggression (BGLHA), to evaluate impulsivity and aggression, respectively, were employed. When analyses were parsed by individual diagnoses, group-wise correlational analyses yielded a significant positive correlation of Glu/tCr with BIS-11 total score for the BPD participants and a negative correlation for the BPD and the healthy control participants for the BGLHA aggression score with GABA. However, neither correlation was significant for the ADHD group. These results provide some evidence for the role of excitatory and inhibitory neurotransmitters in the pathology of impulsivity and aggression in women with BPD.
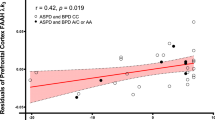
An [11C]CURB PET study [54] that investigated fatty acid amide hydrolase (FAAH), an enzyme of the endocannabinoid system that degrades anandamide and thereby indirectly regulates cannabinoid receptor signaling, examined 16 males with ASPD and 16 male control participants (five with schizophrenia). Results revealed that cerebellar and striatal FAAH expression were inversely related with impulsivity, while cerebellar FAAH density was also negatively associated with assaultive aggressive. These results point to a potential endocannabinoid-lowering process in ASPD that could affect manifestation of impulsivity and aggression in this population.
Finally, one study demonstrated that in 27 violent offenders, gray matter volume in multiple prefrontal regions including superior frontal gyrus and superior orbital gyrus was negatively associated with PCL-R Factor 2 traits [55]. However, further analysis revealed that this effect was mostly driven by Facet 4 traits (antisocial behavior), rather than Facet 3 traits (which includes impulsivity). This study is discussed in further detail in the section on callousness (below).
Taken together, these studies suggest that abnormalities of fronto-temporal-limbic regions are implicated in the impulsivity of BPD and ASPD and may predispose to aggressive behaviors. Neurochemically, alterations in serotonergic and endocannabinoid system signaling pathways may also give rise to impulsivity and aggression in these populations. It should be noted that most of the reviewed studies of impulsivity and aggression in BPD and ASPD used questionnaire-based measures of impulsivity, as opposed to neuropsychological paradigms, to assay these constructs.
Neuroimaging studies investigating risk-taking and aggression
We found two studies linking neural correlates of risk-taking to metrics of aggression, both in incarcerated male offenders. In one of study [56], 49 adult male incarcerated offenders with a mean PCL-R score of 23.5 were administered an intertemporal choice (e.g., delay-discounting) task, while using a mobile fMRI scanner to investigate task-related activation and resting-state functional connectivity. Higher psychopathy (PCL-R score) was associated with stronger subjective value-related striatal activation (within the nucleus accumbens [NAcc]) during inter-temporal choice behavior and with weaker cortico-striatal connectivity (between NAcc and ventromedial prefrontal cortex [vmPFC]), suggesting a potential link between these abnormalities and risky decision-making in personality-disordered men. Further, across all participants, both stronger striatal value-related activation and attenuated cortico-striatal connectivity were associated with a greater total number of convicted crimes. These results suggest that dysregulated cortico-striatal circuits may drive risky decision making across a spectrum of antisociality in men and underscore value-based decision-making as a potential proximal mechanism underlying self-control deficits in disinhibitory syndromes [57, 58].
Another study of violent criminal offenders explored emotion-related mechanisms leading to risky decisions using an fMRI paradigm, where respondents were required to choose between low-risk bonds and high-risk alternatives, such as stocks [59]. While bonds were always a safe choice, stocks could win or lose with varying certainty. All of the offenders met criteria for ASPD. This group was further subdivided into emotionally hypo-reactive offenders (e.g., high PCL-R Factor 1 score and not more than two BPD criteria met; n = 11) and hyper-reactive offenders (e.g., low PCL-R Factor 1 and diagnosis of BPD; n = 12). Thirteen male healthy controls without a criminal or psychiatric history also participated. Results revealed that hypo-reactive offenders differed from healthy controls by exhibiting decreased neural activation in rostral ACC in response to uncertainty and decreased activity in the prefrontal cortex when consistently choosing safe alternatives. There was a positive correlation in hypo-reactive offenders between right inferior frontal gyrus activity preceding a “stock” choice and subscale scores on a questionnaire measuring aggression as well as the number of risk-seeking mistakes, which was interpreted as a measure of behavioral dyscontrol.
Neuroimaging studies investigating callousness/lack of empathy and aggression
One study in 27 violent offenders and 27 healthy controls [55] linked structural brain abnormalities in the offending group to both antisocial traits (PCL-R facets) and to aggression (using Aggression Questionnaire, AQ, and Reactive–Proactive–Aggression Questionnaire, RPQ) [60]. Total and sub-scale scores of these measures were correlated with gray matter volumes (GMVs), using VBM-based brain morphometry, in the offenders. For PCL-R scores, as noted in “impulsivity” section above, findings demonstrated a link specifically between prefrontal GMV and Facet 4 antisocial behavior traits, such as juvenile delinquency and recidivism. For Facet 2 traits, which include “callousness/lack of empathy,” there was no correlation. For aggression scores, only one sub-scale of the trait aggression scales correlated significantly with GMV in offenders. Specifically, RPQ reactive aggression was negatively linked with GMV in the right middle and superior temporal gyrus. Together, these findings suggest a link between prefrontal GMV and antisocial behavior, potentially mediated through reactive aggression. They do not provide support for the contribution of any GMV deficits and callousness/lack of empathy, which has typically been linked to proactive aggression. This is discussed further in the limitations section below. We did not find any further studies in our included subject groups that linked a neuroimaging metric to aggression and also to callous-unemotional traits or lack of empathy.
Discussion
To explore how trait-based approaches to neuroimaging research in BPD and ASPD have progressed since publication of DSM-5, we conducted a systematic review of neuroimaging studies investigating key traits linked to aggression across these disorders. While a lack of methodological consistency in the field remains and limits the scope of our findings, our study identified some important considerations for future work.
First, evidence from studies identified in our review suggests that anger/hostility associated with alterations in the interplay between prefrontal (dlPFC, vlPFC, OFC, and mPFC) and subcortical regions (primarily the amygdala) could be a common factor explaining aggressive reactions in response to perceived interpersonal threat or provocation. Interestingly, findings indicate that a proneness to act out aggressively may be linked to a reduced differentiation (at a neural and behavioral level) between threatening and non-threatening interpersonal cues, in line with the hypothesis of a hostile filter that biases the perception of the entire social environment, thus increasing the likelihood for aggressive encounters. This is also in line with the findings of an earlier PET study, which revealed stronger amygdala responses to high as well as low provocation in individuals with comorbid BPD and IED [61]. However, this hypothesis needs to be tested in a large group of individuals across specific personality disorders and both sexes.
Second, there remains an overall lack of clarity about the respective links between neural correlates of impulsivity and aggression. This is surprising, given prior evidence suggesting that impulsive behavior and reactive aggression may share common neural underpinnings [62]. One potential explanation is that in the majority of studies linking impulsivity to aggression in this review, questionnaire-based measures of impulsivity were employed. This may be critical, because neuropsychological testing of impulsivity does not uniformly overlap with impulsivity measured via self-report [52]. In fact, some authors argue that data are lacking for a relationship between behavioral impulsivity and self-reported impulsivity, possibility pointing to different constructs [63]. Whether a lack of neuropsychological measures testing impulsivity in these studies has relevance for understanding the neural correlates of aggression in BPD and ASPD is currently unknown. However, from the reviewed studies, alterations in the structure and function of fronto-temporal-limbic regions are implicated in the impulsivity of BPD and ASPD that may give rise to aggressive behaviors. Furthermore, there may be a role for certain neuromodulatory systems, such as the serotonergic or endocannabinoid signaling systems, in connecting impulsive behavior to aggressive responding. This is also consistent with the results of a row of earlier studies in individuals with IED, which revealed a role for the serotonergic system in impulsive aggression [64, 65].
Another potential explanation for this lack of clarity is the conceptual overlap between impulsivity and risk taking. This may explain in part why our search yielded so few studies specifically examining links between risk-taking and aggression. Whereas impulsivity may reflect acting on the spur of the moment in response to immediate stimuli, acting on a momentary basis without consideration of outcomes, or difficulty establishing and following plans, risk-taking may involve poor representation of the degree of risk, especially in situations where the degree of risk gradually increases combined with the magnitude of a potential favorable outcome when the precise odds of negative outcomes are unknown or not explicit. However, some imaging reports in healthy populations have argued for a clear dissociation between high-risk behavior tendency as a construct distinct from that of impulsivity [66]. We propose that a clearer demarcation in the AMPD system between impulsivity and risk-taking could shed further light on possible unique neural correlates between these traits and aggression in BPD and ASPD.
Third, we found a notable lack of studies that investigated potential neuroimaging correlates for both callousness/lack of empathy and aggression. This was also surprising, considering considerable previous evidence suggesting a neurobiological component to callousness [67,68,69] and the putative link between callousness and aggression, particularly proactive aggression [29]. While a detailed neurobiological model of reactive aggression has been previously outlined [70], a corresponding model for proactive aggression has not yet emerged. However, the integrated emotion system (IES) model [71] offers one potential explanation, in those high on trait callousness/lack of empathy. First, reduced amygdala functioning, which leads to impaired processing of fear, may result in a lack of deterrence from harming others to gain advantage. Second, decision-making deficits driven by striatal dysfunction and other reward-related circuitry such as vmPFC [72] may lead to those high on trait callousness/lack of empathy to take pleasure in causing harm to others. These deficits are seen in individuals with ASPD and psychopathy, in whom callousness/lack of empathy and proactive aggression is characteristic [29, 73]. However, there is some evidence that callousness may also play a role in reactive aggression. One PET study with patients with IED characterized by high levels of impulsive aggressive outbursts found trait callousness exhibiting a significant positive correlation with the serotonin transporter availability in the ACC [74]. Future work will benefit from examination of these potential neurobiological underpinnings of aggression, for example, by linking performance on empathy-inducing fMRI tasks with behavioral measures of reactive and proactive aggression.
Our review identified further gaps in the existing literature. First, the included studies used a variety of different measures for aggression, ranging from different self-report trait questionnaires, interviews assessing aggressive behavior within the past weeks, to aggressive responses in experiments. Although acceptable reliability has been shown for most of these measures, their ecological validity remains questionable. There are several reasons for this: i) the correlations between different measures of aggression are often small to moderate; ii) most of these measures are subject to social desirability effects; iii) measuring aggression in a highly standardized yet ecologically valid experiment is a particular challenge in a neuroimaging setting; and iv) most of these measures do not provide information about which particular situation a particular person acts out aggressively in real life. Hence, future studies combining neuroimaging methods with ecological momentary assessment are needed.
Second, there was a marked lack of specificity about forms of aggression in most studies in our review. This is especially important as previous evidence suggests that reactive aggression is more likely be associated with anger and impulsivity [62] and proactive (e.g., instrumental) aggression may be distinctly linked to severity of callousness/lack of empathy [75, 76]. Arguably, new paradigms are required that are better able to differentiate between these subtypes of aggression and also between different triggers for aggression (e.g., provocation, frustration, threat).
Third, none of the studies in our review investigated more than one of the personality traits potentially related to aggression within a single sample. This limits inferences on the specificity of findings from individual studies. It also precludes investigation of the interplay between these specific traits in a causative model of aggression. Future studies will benefit from a principled approach to exploring links between the relative contributions of individual traits (selected based on prior work demonstrating links to aggression) - and their neural underpinnings - to aggression. Analytical models that can explore whether particular traits, or combinations of traits, mediate the correlation between neural signatures of aggression, or vice versa, would be particularly beneficial. Such studies will likely require more specific measures of aggression, as well as large and heterogeneous samples of aggressive individuals with personality disorders [13].
Fourth, with few exceptions, the majority of neuroimaging studies of aggression in BPD samples involve women only, while neuroimaging studies of aggression in ASPD and psychopathy focus on men. To some extent, these patterns reflect the prevalence of each condition by sex under certain scenarios. For example, females with BPD are over-represented in clinical settings [77], while ASPD is 5–7 more times common in males than females [78]. Still, it cannot be assumed that the neurobiology of aggression in ASPD males is the same as in females, or conversely, that neuroimaging findings in female BPD patients are the same as in male BPD subjects [37]. A more fulsome understanding of sex differences and their underlying neurobiology may be important in developing sex-specific treatment programmes. For example, limited research suggests that males with BPD experience a greater reduction in physical aggression and develop enhanced anger management skills compared to females with BPD in a dialectical behavior therapy program specifically modified for corrections [79].
Future directions
Previous reviews of clinical studies using AMPD have suggested that this model demonstrates acceptable interrater reliability, largely consistent latent structures, substantial convergence with relevant external measures, evidence for incremental validity when controlling for categorical PD diagnoses [80], and clinical utility [81]. Our review has highlighted that applying a trait-based approach to the neurocognitive basis of personality disorders may additionally yield mechanistic insights. However, future work should acknowledge potential limitations of this approach. Other studies have demonstrated high correlation of criterion A and B [80], correlation of criterion A with both axis I and axis II disorders [82], and the finding that traits (criterion B) account for substantially more unique variance in DSM-5 Section II PDs than does personality impairment (criterion A) [82, 83]. Moreover, many clinicians and researchers continue to have reservations about several aspects of application of AMPD, including the communicative value between clinicians and their patients’ families, the feasibility of the model’s application, and the model’s ability to translate into treatment modalities [81]. Future studies should address these issues and seek to demonstrate added value over categorical approaches, in order to justify a wider shift to the dimensional approach of AMPD.
Furthermore, some consideration should be given to the potentially loaded and negatively-valenced descriptors of certain personality traits. Consistent terminology about antisocial symptom domains remains important and has utility in inter-professional communication [84]. However, a shift to routine use of the terms “callousness” and “hostility”, especially in younger populations, may be deemed unacceptable by patient groups and their advocates. Notably, DSM-5 uses the alternate term “Limited Prosocial Emotions,” in specifier criteria for conduct disorder (the precursor of ASPD). Careful development and application of terminology will likely have a role in developing a consensus-based empirical approach in this area. Additionally, an overly reductionist portrayal of these symptoms as discrete entities, emerging from distinct neurobiological deficits, and which are relatively immutable, is likely to be misguided. For instance, emerging evidence in youth populations suggests that callous-unemotional traits are themselves heterogeneous [85, 86] and vary throughout personality development [87, 88]. Longitudinal neuroimaging data that illustrate consistent patterns of disrupted brain development will be an important further development [89].
Methodological issues also warrant further consideration. The lack of a consistent approach to inclusion criteria and stratification of groups of antisocial and violent offenders in studies could be addressed by consensus approach to defining both categorical measurements (e.g., DSM-5 criteria for ASPD and PCL-R for psychopathy) and symptom domains (e.g., using DSM-5 criteria for Limited Prosocial Emotions). Second, since aggression is a complex phenomenon, future studies will need to include multi-methodological designs: hormones and physiological measures indicating the level of peripheral arousal should accompany neuroimaging and self-reports. Several studies have suggested that arousal, for example, resting state heart rate [90] shows a moderate negative correlation with violent behavior. Similarly, associations between testosterone and cortisol levels and responses to stress or provocation and aggression need to be taken into account. Other methods besides neuroimaging, such as electroencephalography, may provide important insights in the timing of cortical processing and the level of automacy of the above discussed processes. Ecological momentary assessments might be useful to acquire information about the situations in which an individual acts out aggressively and how this is related to maladaptive trait profiles.
Conclusions
This systematic review examined trait-based approaches to aggression in neuroimaging research in BPD and ASPD published since the introduction of AMPD. While there were relatively few neuroimaging studies examining AMPD traits relevant to aggression in BPD and ASPD, several key themes emerged. First, a variety of different measures for aggression exist, but studies combining neuroimaging methods with ecological momentary assessment are needed to better understand under what situations particular individuals act out aggressively in real life. Second, very few of the studies differentiate between proactive and reactive forms of aggression, which has relevance for understanding how subtypes of aggression relate to AMPD traits. Third, the existing neuroimaging studies are limited to the study of only one particular trait in relation to aggression, when in reality most individuals will likely endorse multiple AMPD traits. Fourth, most studies of BPD focus on females, while those of ASPD sample males. This lack of heterogeneity makes it difficult to parse the neuroimaging markers of aggression in male BPD patients and female ASPD subjects. We have also highlighted methodological inconsistencies across the existing literature and emphasized the importance of a consistent approach to categorical and trait specification. We conclude that multi-methodological designs incorporating a range of biomarkers hold the most promise for understanding how a relatively new maladaptive trait model of personality disorders can better inform on the biological underpinnings of aggression in BPD and ASPD.
References
Yu R, Geddes JR, Fazel S. Personality disorders, violence, and antisocial behavior: a systematic review and meta-regression analysis. J Personal Disord. 2012;26:775.
Newhill CE, Eack SM, Mulvey EP. Violent behavior in borderline personality. J Personal Disord. 2009;23:541.
Robitaille M-P, Checknita D, Vitaro F, Tremblay RE, Paris J, Hodgins S. A prospective, longitudinal, study of men with borderline personality disorder with and without comorbid antisocial personality disorder. Borderline Personal Disord Emot Dysregul. 2017;4:1–13.
Arola R, Antila H, Riipinen P, Hakko H, Riala K, Kantojärvi L. Borderline personality disorder associates with violent criminality in women: a population based follow-up study of adolescent psychiatric inpatients in Northern Finland. Forensic Sci Int. 2016;266:389–95.
Becker DF, Grilo CM, Edell WS, McGlashan TH. Comorbidity of borderline personality disorder with other personality disorders in hospitalized adolescents and adults. Am J Psychiatry. 2000;157:2011–16.
Coid J, Moran P, Bebbington P, Brugha T, Jenkins R, Farrell M, et al. The co‐morbidity of personality disorder and clinical syndromes in prisoners. Crim Behav Ment Health. 2009;19:321–33.
Freestone M, Howard R, Coid JW, Ullrich S. Adult antisocial syndrome co‐morbid with borderline personality disorder is associated with severe conduct disorder, substance dependence and violent antisociality. Personal Ment Health. 2013;7:11–21.
Howard RC, Khalifa N, Duggan C. Antisocial personality disorder comorbid with borderline pathology and psychopathy is associated with severe violence in a forensic sample. J Forensic Psychiatry Psychol. 2014;25:658–72.
Gibbon S, Khalifa NR, Cheung NH, Völlm BA, McCarthy L. Psychological interventions for antisocial personality disorder. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD007668.pub3.
Khalifa NR, Gibbon S, Völlm BA, Cheung NH, McCarthy L. Pharmacological interventions for antisocial personality disorder. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD007667.pub3.
Látalová K, Praško J. Aggression in borderline personality disorder. Psychiatr Q. 2010;81:239–51.
Bertsch K, Florange J, Herpertz SC. Understanding brain mechanisms of reactive aggression. Curr psychiatry Rep. 2020;22:1–16.
Blair RJ. The motivation of aggression: a cognitive neuroscience approach and neurochemical speculations. Motiv Sci. 2022;8:106.
Mancke F, Herpertz SC, Bertsch K. Aggression in borderline personality disorder: a multidimensional model. Personal Disord Theory Res Treat. 2015;6:278.
Bertsch K, Hillmann K, Herpertz SC. Behavioral and neurobiological correlates of disturbed emotion processing in borderline personality disorder. Psychopathology. 2018;51:76–82.
Chu J, Zheng K, Yi J. Aggression in borderline personality disorder: a systematic review of neuroimaging studies. Prog Neuro Psychopharmacol Biol Psychiatry. 2022;113:110472.
Winsper C, Marwaha S, Lereya ST, Thompson A, Eyden J, Singh SP. A systematic review of the neurobiological underpinnings of borderline personality disorder (BPD) in childhood and adolescence. Rev Neurosci. 2016;27:827–47.
Blair RJ. Functional MRI studies of antisocial personality disorder. In: Textbook of antisocial personality disorder. American Psychiatric Association Publishing. Washington: DC; 2022. p. 269.
Dugré JR, Radua J, Carignan-Allard M, Dumais A, Rubia K, Potvin S. Neurofunctional abnormalities in antisocial spectrum: a meta-analysis of fMRI studies on Five distinct neurocognitive research domains. Neurosci Biobehav Rev. 2020;119:168–83.
Mancke F, Herpertz SC, Bertsch K. Correlates of aggression in personality disorders: an update. Curr Psychiatry Rep. 2018;20:1–14.
Fanning JR, Keedy S, Berman ME, Lee R, Coccaro EF. Neural correlates of aggressive behavior in real time: a review of fMRI studies of laboratory reactive aggression. Curr Behav Neurosci Rep. 2017;4:138–50.
Leclerc P, Savard C, Vachon DD, Payant M, Lampron M, Tremblay M, et al. Associations between the personality inventory for DSM-5 trait facets and aggression among outpatients with personality disorder: a multimethod study. Compr Psychiatry. 2022;116:152316.
Dougherty DM, Bjork JM, Huckabee HC, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Res. 1999;85:315–26.
Kolla NJ, Meyer JH, Bagby RM, Brijmohan A. Trait anger, physical aggression, and violent offending in antisocial and borderline personality disorders. J forensic Sci. 2017;62:137–41.
González RA, Igoumenou A, Kallis C, Coid JW. Borderline personality disorder and violence in the UK population: categorical and dimensional trait assessment. BMC psychiatry. 2016;16:1–10.
Shorey RC, Brasfield H, Febres J, Stuart GL. The association between impulsivity, trait anger, and the perpetration of intimate partner and general violence among women arrested for domestic violence. J Interpers Violence. 2011;26:2681–97.
Azevedo J, Vieira-Coelho M, Castelo-Branco M, Coelho R, Figueiredo-Braga M. Impulsive and premeditated aggression in male offenders with antisocial personality disorder. PLoS One. 2020;15:e0229876.
Romero-Martínez Á, Lila M, Moya-Albiol L. Empathy impairments in intimate partner violence perpetrators with antisocial and borderline traits: A key factor in the risk of recidivism. Violence Vict. 2016;31:347–60.
Hare RD. The psychopathy checklist–Revised. Multi Health Systems. Toronto, ON; 2003.
Bertsch K, Buades-Rotger M, Krauch M, Ueltzhöffer K, Kleindienst N, Herpertz SC, et al. Abnormal processing of interpersonal cues during an aggressive encounter in women with borderline personality disorder: neural and behavioral findings. J Psychopathol Clin Sci. 2022;131:493–506.
Beyer F, Munte TF, Gottlich M, Kramer UM. Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb Cortex. 2015;25:3057–63.
Buades-Rotger M, Engelke C, Beyer F, Keevil BG, Brabant G, Krämer UM. Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Sci Rep. 2016;6:1–14.
Bertsch K, Krauch M, Roelofs K, Cackowski S, Herpertz SC, Volman I. Out of control? Acting out anger is associated with deficient prefrontal emotional action control in male patients with borderline personality disorder. Neuropharmacology. 2019;156:107463.
Bertsch K, Roelofs K, Roch PJ, Ma B, Hensel S, Herpertz SC, et al. Neural correlates of emotional action control in anger-prone women with borderline personality disorder. J Psychiatry Neurosci. 2018;43:161–70.
Volman I, von Borries AK, Bulten BH, Verkes RJ, Toni I, Roelofs K. Testosterone modulates altered prefrontal control of emotional actions in psychopathic offenders(1,2,3). eNeuro. 2016;3:ENEURO.0107–15.2016.
Morandotti N, Dima D, Jogia J, Frangou S, Sala M, Vidovich GZ, et al. Childhood abuse is associated with structural impairment in the ventrolateral prefrontal cortex and aggressiveness in patients with borderline personality disorder. Psychiatry Res. 2013;213:18–23.
Herpertz SC, Nagy K, Ueltzhöffer K, Schmitt R, Mancke F, Schmahl C, et al. Brain mechanisms underlying reactive aggression in borderline personality disorder—sex matters. Biol Psychiatry. 2017;82:257–66.
Neukel C, Bertsch K, Wenigmann M, Spiess K, Krauch M, Steinmann S, et al. A mechanism-based approach to anti-aggression psychotherapy in borderline personality disorder: group treatment affects amygdala activation and connectivity. Brain Sci. 2021;11:1627.
da Cunha-Bang S, Fisher PM, Hjordt LV, Holst K, Knudsen GM. Amygdala reactivity to fearful faces correlates positively with impulsive aggression. Soc Neurosci. 2019;14:162–72.
da Cunha-Bang S, Fisher PM, Hjordt LV, Perfalk E, Persson Skibsted A, Bock C, et al. Violent offenders respond to provocations with high amygdala and striatal reactivity. Soc Cogn Affect Neurosci. 2017;12:802–10.
Tonnaer F, Siep N, van Zutphen L, Arntz A, Cima M. Anger provocation in violent offenders leads to emotion dysregulation. Sci Rep. 2017;7:3583.
Siep N, Tonnaer F, van de Ven V, Arntz A, Raine A, Cima M. Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. 2019;13:1311–23.
Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2014;16:1–13.
Schmahl C, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40:419–27.
Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46:516–25.
Soloff PH, Abraham K, Burgess A, Ramaseshan K, Chowdury A, Diwadkar VA. Impulsivity and aggression mediate regional brain responses in Borderline Personality Disorder: An fMRI study. Psychiatry Res Neuroimaging. 2017;260:76–85.
Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–41.
Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–42.
Soloff P, White R, Diwadkar VA. Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Res Neuroimaging. 2014;222:131–39.
Soloff PH, Chiappetta L, Mason NS, Becker C, Price JC. Effects of serotonin-2A receptor binding and gender on personality traits and suicidal behavior in borderline personality disorder. Psychiatry Res Neuroimaging. 2014;222:140–48.
Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC. Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Res. 2010;181:77–84.
Wagner G, Krause-Utz A, de la Cruz F, Schumann A, Schmahl C, Baer K-J. Resting-state functional connectivity of neurotransmitter producing sites in female patients with borderline personality disorder. Prog Neuro Psychopharmacol Biol Psychiatry. 2018;83:118–26.
Ende G, Cackowski S, Van Eijk J, Sack M, Demirakca T, Kleindienst N, et al. Impulsivity and aggression in female BPD and ADHD Patients: Association with ACC glutamate and GABA concentrations. Neuropsychopharmacology. 2016;41:410–8.
Kolla NJ, Boileau I, Karas K, Watts JJ, Rusjan P, Houle S, et al. Lower amygdala fatty acid amide hydrolase in violent offenders with antisocial personality disorder: an [11C] CURB positron emission tomography study. Transl Psychiatry. 2021;11:1–11.
Hofhansel L, Weidler C, Votinov M, Clemens B, Raine A, Habel U. Morphology of the criminal brain: gray matter reductions are linked to antisocial behavior in offenders. Brain Struct Funct. 2020;225:2017–28.
Hosking JG, Kastman EK, Dorfman HM, Samanez-Larkin GR, Baskin-Sommers A, Kiehl KA, et al. Disrupted prefrontal regulation of striatal subjective value signals in psychopathy. Neuron. 2017;95:221–31.e4.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–21.
Baskin-Sommers A, Stuppy-Sullivan AM, Buckholtz JW. Psychopathic individuals exhibit but do not avoid regret during counterfactual decision making. Proc Natl Acad Sci. 2016;113:14438–43.
Prehn K, Schlagenhauf F, Schulze L, Berger C, Vohs K, Fleischer M, et al. Neural correlates of risk taking in violent criminal offenders characterized by emotional hypo-and hyper-reactivity. Soc Neurosci. 2013;8:136–47.
Raine A, Dodge K, Loeber R, Gatzke‐Kopp L, Lynam D, Reynolds C, et al. The reactive–proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behav Off J Int Soc Res Aggression. 2006;32:159–71.
New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, et al. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry. 2009;66:1107–14.
Bowyer CB, Joyner KJ, Latzman RD, Venables NC, Foell J, Patrick CJ. A model-based strategy for interfacing traits of the DSM-5 AMPD with neurobiology. J Pers Disord. 2020;34:586–608.
Stahl C, Voss A, Schmitz F, Nuszbaum M, Tuscher O, Lieb K, et al. Behavioral components of impulsivity. J Exp Psychol Gen. 2014;143:850–86.
Perez-Rodriguez MM, Hazlett EA, Rich EL, Ripoll LH, Weiner DM, Spence N, et al. Striatal activity in borderline personality disorder with comorbid intermittent explosive disorder: sex differences. J Psychiatr Res. 2012;46:797–804.
Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C] McN 5652. Am J Psychiatry. 2005;162:915–23.
Brown MR, Benoit JR, Juhás M, Lebel RM, MacKay M, Dametto E, et al. Neural correlates of high-risk behavior tendencies and impulsivity in an emotional Go/NoGo fMRI task. Front Syst Neurosci. 2015;9:24.
De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: increased grey matter in boys with conduct problems and callousunemotional traits. Brain. 2009;132:843–52.
Sebastian CL, De Brito SA, McCrory EJ, Hyde ZH, Lockwood PL, Cecil CA, et al. Grey matter volumes in children with conduct problems and varying levels of callous-unemotional traits. J Abnorm child Psychol. 2016;44:639–49.
Viding E, Frick PJ, Plomin R. Aetiology of the relationship between callous-unemotional traits and conduct problems in childhood. Br J Psychiatry. 2007;190:s33–8.
Blair R. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br J Psychol. 2010;101:383–99.
Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005;17:865–91.
Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Mol Psychiatry. 2009;14:5.
Cornell DG, Warren J, Hawk G, Stafford E, Oram G, Pine D. Psychopathy in instrumental and reactive violent offenders. J Consulting Clin Psychol. 1996;64:783.
van de Giessen E, Rosell DR, Thompson JL, Xu X, Girgis RR, Ehrlich Y, et al. Serotonin transporter availability in impulsive aggressive personality disordered patients: A PET study with [11C] DASB. J Psychiatr Res. 2014;58:147–54.
Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J Abnorm Child Psychol. 2003;31:457–70.
Fanti KA, Frick PJ, Georgiou S. Linking callous-unemotional traits to instrumental and non-instrumental forms of aggression. J Psychopathol Behav Assess. 2009;31:285.
Sansone RA, Sansone LA. Gender patterns in borderline personality disorder. Innov Clin Neurosci. 2011;8:16–20.
Hamdi NR, Iacono WG. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychol Med. 2014;44:315–24.
Shelton D, Sampl S, Kesten KL, Zhang W, Trestman RL. Treatment of impulsive aggression in correctional settings. Behav Sci Law. 2009;27:787–800.
Zimmermann J, Kerber A, Rek K, Hopwood CJ, Krueger RF. A brief but comprehensive review of research on the alternative DSM-5 model for personality disorders. Curr psychiatry Rep. 2019;21:1–19.
Milinkovic MS, Tiliopoulos N. A systematic review of the clinical utility of the DSM–5 section III alternative model of personality disorder. Personal Disord Theory Res Treat. 2020;11:377.
Sleep CE, Lynam DR, Widiger TA, Crowe ML, Miller JD. An evaluation of DSM–5 Section III personality disorder Criterion A (impairment) in accounting for psychopathology. Psychological Assess. 2019;31:1181.
Few LR, Miller JD, Rothbaum AO, Meller S, Maples J, Terry DP, et al. Examination of the Section III DSM-5 diagnostic system for personality disorders in an outpatient clinical sample. J Abnorm Psychol. 2013;122:1057.
Scott S. Debate:‘A rose by any other name’would smell as sweet–myths peddled about the ills of diagnosing conduct disorders. Child Adolesc Ment Health. 2022;27:302–04.
Cecil CA, McCrory EJ, Barker ED, Guiney J, Viding E. Characterising youth with callous–unemotional traits and concurrent anxiety: evidence for a high-risk clinical group. Eur Child Adolesc Psychiatry 2018;27:885–98.
Meehan AJ, Maughan B, Cecil CA, Barker ED. Interpersonal callousness and co-occurring anxiety: developmental validity of an adolescent taxonomy. J Abnorm Psychol. 2017;126:225.
Fanti KA, Colins OF, Andershed H, Sikki M. Stability and change in callous-unemotional traits: longitudinal associations with potential individual and contextual risk and protective factors. Am J Orthopsychiatry. 2017;87:62.
Fontaine NM, McCrory EJ, Boivin M, Moffitt TE, Viding E. Predictors and outcomes of joint trajectories of callous–unemotional traits and conduct problems in childhood. J Abnorm Psychol. 2011;120:730.
Raine A. Antisocial personality as a neurodevelopmental disorder. Annu Rev Clin Psychol. 2018;14:259–89.
De Looff PC, Cornet LJ, De Kogel CH, Fernández-Castilla B, Embregts PJ, Didden R, et al. Heart rate and skin conductance associations with physical aggression, psychopathy, antisocial personality disorder and conduct disorder: An updated meta-analysis. Neuroscience & Biobehavioral Reviews. 2021.
Costa PT Jr., McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50.
Coccaro EF. Psychiatric comorbidity in intermittent explosive disorder. Intermittent Explosive Disorder. Elsevier; 2019. p. 67–84.
APA APA. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Krueger RF, Derringer J, Markon KE, Watson D, Skodol AE. Initial construction of a maladaptive personality trait model and inventory for DSM-5. Psychol Med. 2012;42:1879–90.
Hopwood CJ, Kotov R, Krueger RF, Watson D, Widiger TA, Althoff RR, et al. The time has come for dimensional personality disorder diagnosis. Personal Ment health. 2018;12:82–86.
Clark LA. Assessment and diagnosis of personality disorder: perennial issues and an emerging reconceptualization. Annu Rev Psychol. 2007;58:227–57.
Krueger RF, Markon KE. The role of the DSM-5 personality trait model in moving toward a quantitative and empirically based approach to classifying personality and psychopathology. Annu Rev Clin Psychol. 2014;10:477–501.
Widiger TA, Trull TJ. Plate tectonics in the classification of personality disorder: shifting to a dimensional model. Am Psychologist. 2007;62:71.
Bornstein RF, Natoli AP. Clinical utility of categorical and dimensional perspectives on personality pathology: a meta-analytic review. Personal Disord. 2019;10:479–90.
Widiger TA, Simonsen E, Krueger R, Livesley WJ, Verheul R. Personality disorder research agenda for the DSM–V. J Personal Disord. 2005;19:315.
Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169:577–88.
Coccaro EF, Shima CK, Lee RJ. Comorbidity of personality disorder with intermittent explosive disorder. J Psychiatr Res. 2018;106:15–21.
Coccaro EF, Fitzgerald DA, Lee R, McCloskey M, Phan KL. Frontolimbic morphometric abnormalities in intermittent explosive disorder and aggression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:32–38.
Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–57.
Buss AH, Perry M. The aggression questionnaire. J Personal Soc Psychol. 1992;63:452.
Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69:1153–59.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505.
Lee R, Arfanakis K, Evia AM, Fanning J, Keedy S, Coccaro EF. White matter integrity reductions in intermittent explosive disorder. Neuropsychopharmacology. 2016;41:2697–703.
Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15:351–71.
Acknowledgements
The authors would like to thank Information Specialist, Naomi Thorpe BSc, MSc, for her valuable contribution to the literature search process.
Author information
Authors and Affiliations
Contributions
NJK, JT, and KB all contributed to the concept and design of the study. NJK, JT, and KB all contributed to the acquisition, analysis, and interpretation of the data. All authors drafted the article and revised it critically for important intellectual content, and NJK, JT, and KB all approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kolla, N.J., Tully, J. & Bertsch, K. Neural correlates of aggression in personality disorders from the perspective of DSM-5 maladaptive traits: a systematic review. Transl Psychiatry 13, 330 (2023). https://doi.org/10.1038/s41398-023-02612-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02612-1