Abstract
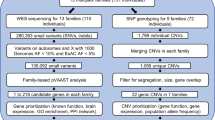
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder characterized by repetitive motor movements and vocal tics. The clinical manifestations of TS are complex and often overlap with other neuropsychiatric disorders. TS is highly heritable; however, the underlying genetic basis and molecular and neuronal mechanisms of TS remain largely unknown. We performed whole-exome sequencing of a hundred trios (probands and their parents) with detailed records of their clinical presentations and identified a risk gene, ASH1L, that was both de novo mutated and associated with TS based on a transmission disequilibrium test. As a replication, we performed follow-up targeted sequencing of ASH1L in additional 524 unrelated TS samples and replicated the association (P value = 0.001). The point mutations in ASH1L cause defects in its enzymatic activity. Therefore, we established a transgenic mouse line and performed an array of anatomical, behavioral, and functional assays to investigate ASH1L function. The Ash1l+/− mice manifested tic-like behaviors and compulsive behaviors that could be rescued by the tic-relieving drug haloperidol. We also found that Ash1l disruption leads to hyper-activation and elevated dopamine-releasing events in the dorsal striatum, all of which could explain the neural mechanisms for the behavioral abnormalities in mice. Taken together, our results provide compelling evidence that ASH1L is a TS risk gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request. All relevant data will be deposited into a public repository and accession codes will be available before publication.
References
Groth C, Mol Debes N, Rask CU, Lange T, Skov L. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry. 2017;56:304–12.
Cavanna AE, Termine C. Tourette syndrome. Adv Exp Med Biol. 2012;724:375–83.
Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65:461–72.
Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30:221–8.
Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–33.
Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2:68–87.
Liu S, Zheng L, Zheng X, Zhang X, Yi M, Ma X. The subjective quality of life in young people with Tourette syndrome in China. J Atten Disord. 2017;21:426–32.
Huisman-van Dijk HM, Schoot R, Rijkeboer MM, Mathews CA, Cath DC. The relationship between tics, OC, ADHD and autism symptoms: a cross-disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Res. 2016;237:138–46.
Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Dev Psychopathol. 2005;17:415–45.
Channon S, Gunning A, Frankl J, Robertson MM. Tourette’s syndrome (TS): cognitive performance in adults with uncomplicated TS. Neuropsychology. 2006;20:58–65.
Pauls DL, Cohen DJ, Heimbuch R, Detlor J, Kidd KK. Familial pattern and transmission of Gilles de la Tourette syndrome and multiple tics. Arch Gen Psychiatry. 1981;38:1091–3.
Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. Am J Hum Genet. 1991;48:154–63.
Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815–20.
Hyde TM, Aaronson BA, Randolph C, Rickler KC, Weinberger DR. Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology. 1992;42(3 Part 1):652–8.
Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800.
Liu S, Cui J, Zhang X, Wu W, Niu H, Ma X, et al. Variable number tandem repeats in dopamine receptor D4 in Tourette’s syndrome. Mov Disord. 2014;29:1687–91.
Yoon DY, Rippel CA, Kobets AJ, Morris CM, Lee JE, Williams PN, et al. Dopaminergic polymorphisms in Tourette syndrome: association with the DAT gene (SLC6A3). Am J Med Genet B. 2007;144B:605–10.
Dehning S, Muller N, Matz J, Bender A, Kerle I, Benninghoff J, et al. A genetic variant of HTR2C may play a role in the manifestation of Tourette syndrome. Psychiatr Genet. 2010;20:35–38.
Moya PR, Wendland JR, Rubenstein LM, Timpano KR, Heiman GA, Tischfield JA, et al. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Mov Disord. 2013;28:1263–70.
Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, et al. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatr Genet. 2011;21:90–97.
Dong S, Walker MF, Carriero NJ, DiCola M, Willsey AJ, Ye AY, et al. De novo insertions and deletions of predominantly paternal origin are associated with autism spectrum disorder. Cell Rep. 2014;9:16–23.
Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5.
O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9.
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–50.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41.
Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–33.
Willsey AJ, Fernandez TV, Yu D, King RA, Dietrich A, Xing J, et al. De novo coding variants are strongly associated with Tourette disorder. Neuron. 2017;94:486–499 e489.
Huang AY, Yu D, Davis LK, Sul JH, Tsetsos F, Ramensky V, et al. Rare copy number variants in NRXN1 and CNTN6 increase risk for Tourette syndrome. Neuron. 2017;94:1101–1111 e1107.
Krumm N, Turner TN, Baker C, Vives L, Mohajeri K, Witherspoon K, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–8.
He Z, O’Roak BJ, Smith JD, Wang G, Hooker S, Santos-Cortez RL, et al. Rare-variant extensions of the transmission disequilibrium test: application to autism exome sequence data. Am J Hum Genet. 2014;94:33–46.
Singer HS, Szymanski S, Giuliano J, Yokoi F, Dogan AS, Brasic JR, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159:1329–36.
Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–51.
Baldan LC, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90.
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15.
Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21.
Stessman HA, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet. 2017;49:515–26.
de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367:1921–9.
Shen W, Krautscheid P, Rutz AM, Bayrak-Toydemir P, Dugan SL. De novo loss-of-function variants of ASH1L are associated with an emergent neurodevelopmental disorder. Eur J Med Genet. 2019;62:55–60.
Faundes V, Newman WG, Bernardini L, Canham N, Clayton-Smith J, Dallapiccola B, et al. Histone lysine methylases and demethylases in the landscape of human developmental disorders. Am J Hum Genet. 2018;102:175–87.
Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–60.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303.
Huang Z, Rustagi N, Veeraraghavan N, Carroll A, Gibbs R, Boerwinkle E, et al. A hybrid computational strategy to address WGS variant analysis in >5000 samples. BMC Bioinforma. 2016;17:361.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26.
Zhu T, Liang C, Li D, Tian M, Liu S, Gao G, et al. Histone methyltransferase Ash1L mediates activity-dependent repression of neurexin-1alpha. Sci Rep. 2016;6:26597.
Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Mol Psychiatry. 2002;7:617–25. 524
Xie H, Liu Y, Zhu Y, Ding X, Yang Y, Guan JS. In vivo imaging of immediate early gene expression reveals layer-specific memory traces in the mammalian brain. Proc Natl Acad Sci USA. 2014;111:2788–93.
Ding X, Liu S, Tian M, Zhang W, Zhu T, Li D, et al. Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat Neurosci. 2017;20:690–9.
Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol. 2010;6:e1001025.
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5.
Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7 20.
Mele M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, et al. Human genomics. The human transcriptome across tissues and individuals. Science. 2015;348:660–5.
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59.
Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60.
Sungur AO, Vorckel KJ, Schwarting RK, Wohr M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context. J Neurosci Methods. 2014;234:92–100.
Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. 591–602
Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–9.
Xu M, Li L, Ohtsu H, Pittenger C. Histidine decarboxylase knockout mice, a genetic model of Tourette syndrome, show repetitive grooming after induced fear. Neurosci Lett. 2015;595:50–53.
Bloch MH. Emerging treatments for Tourette’s disorder. Curr Psychiatry Rep. 2008;10:323–30.
Du JC, Chiu TF, Lee KM, Wu HL, Yang YC, Hsu SY, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51:255–64.
Kurlan R. Clinical practice. Tourette’s syndrome. N Engl J Med. 2010;363:2332–8.
McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain. 2009;132(Part 8):2125–38.
Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, et al. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135(Part 6):1926–36.
Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91.
Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011;26:1149–56.
Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 2018;174:481–496.e419.
Eram MS, Kuznetsova E, Li F, Lima-Fernandes E, Kennedy S, Chau I, et al. Kinetic characterization of human histone H3 lysine 36 methyltransferases, ASH1L and SETD2. Biochim Biophys Acta. 2015;1850:1842–8.
Baron-Cohen S, Mortimore C, Moriarty J, Izaguirre J, Robertson M. The prevalence of Gilles de la Tourette’s syndrome in children and adolescents with autism. J Child Psychol Psychiatry. 1999;40:213–8.
Burd L, Li Q, Kerbeshian J, Klug MG, Freeman RD. Tourette syndrome and comorbid pervasive developmental disorders. J Child Neurol. 2009;24:170–5.
Stern JS, Robertson MM. Tics associated with autistic and pervasive developmental disorders. Neurol Clin. 1997;15:345–55.
Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism. 2007;11:19–28.
Hou P, Huang C, Liu CP, Yang N, Yu T, Yin Y, et al. Structural insights into stimulation of Ash1L’s H3K36 methyltransferase activity through Mrg15 binding. Structure (London, England: 1993). 2019;27:837–845.e833.
Brinkmeier ML, Geister KA, Jones M, Waqas M, Maillard I, Camper SA. The histone methyltransferase gene absent, small, or homeotic discs-1 like is required for normal hox gene expression and fertility in mice. Biol Reprod. 2015;93:121.
Acknowledgements
We thank Richard A Gibbs, Huda Zoghbi, and Sau Wai Cheung for constructive suggestions. We are grateful to all of the patients and their families for participating in this study. We thank Dr. Yulong Li for the DA sensor virus and Dr. Yi Qian, Dr. Lianhui Zhu and Dr. Haopeng Wang for suggestions on ELISA assay. The work is supported by grants from the National Natural Science Foundation (NSFC) (81371499, 31671104, 81471365, 31970903, and 31371059), the National Key Research and Development Program of China (2016YFC1000307), National Basic Research Program of China (2013CB835100), Beijing Municipal Education Commission “scientific research base - technology innovation platform - the construction of major brain disease laboratory platform” and “subject group - clinical medicine” (BIBD-PXM2013_014226_07_000084), and Beijing Natural Science Foundation (7132083). Shandong Provincial Natural Science Foundation of China (ZR2019PH072). The work was partially funded by the NSFC and the German Research Foundation (DFG) in project Cross model Learning, NSFC (61621136008)/DGF TRR-169 to J-SG. JL and FY were supported by the National Institutes of Health and National Human Genome Research Institute (R01HG008115 awarded to FY). Dr. Schaaf’s work is generously supported by the Joan and Stanford Alexander Family.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, S., Tian, M., He, F. et al. Mutations in ASH1L confer susceptibility to Tourette syndrome. Mol Psychiatry 25, 476–490 (2020). https://doi.org/10.1038/s41380-019-0560-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0560-8
This article is cited by
-
Abnormal H3K4 enzyme catalytic activity and neuronal morphology caused by ASH1L mutations in individuals with Tourette syndrome
European Child & Adolescent Psychiatry (2024)
-
Identity-by-descent analysis of a large Tourette’s syndrome pedigree from Costa Rica implicates genes involved in neuronal development and signal transduction
Molecular Psychiatry (2022)
-
Deficiency of autism risk factor ASH1L in prefrontal cortex induces epigenetic aberrations and seizures
Nature Communications (2021)