Abstract
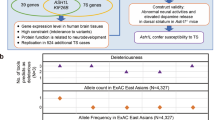
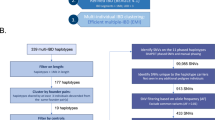
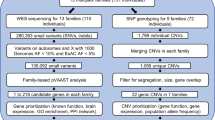
Tourette syndrome (TS) is a highly heritable neuropsychiatric disorder with complex patterns of genetic inheritance. Recent genetic findings in TS have highlighted both numerous common variants with small effects and a few rare variants with moderate or large effects. Here we searched for genetic causes of TS in a large, densely-affected British pedigree using a systematic genomic approach. This pedigree spans six generations and includes 122 members, 85 of whom were individually interviewed, and 53 of whom were diagnosed as “cases” (consisting of 28 with definite or probable TS, 20 with chronic multiple tics [CMT], and five with obsessive-compulsive behaviors [OCB]). A total of 66 DNA samples were available (25 TS, 15 CMT, 4 OCB cases, and 22 unaffecteds) and all were genotyped using a dense single nucleotide polymorphism (SNP) array to identify shared segments, copy number variants (CNVs), and to calculate genetic risk scores. Eight cases were also whole genome sequenced to test whether any rare variants were shared identical by descent. While we did not identify any notable CNVs, single nucleotide variants, indels or repeat expansions of near-Mendelian effect, the most distinctive feature of this family proved to be an unusually high load of common risk alleles for TS. We found that cases within this family carried a higher load of TS common variant risk similar to that previously found in unrelated TS cases. Thus far, the strongest evidence from genetic data for contribution to TS risk in this family comes from multiple common risk variants rather than one or a few variants of strong effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–86.
Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815–20.
Deng H, Gao K, Jankovic J. The genetics of Tourette syndrome. Nat Rev Neurol. 2012;8:203–13.
O’Rourke JA, Scharf JM, Yu D, Pauls DL. The genetics of Tourette syndrome: a review. J Psychosom Res. 2009;67:533–45.
Mataix-Cols D, Isomura K, Perez-Vigil A, Chang Z, Ruck C, Larsson KJ, et al. Familial Risks of Tourette syndrome and chronic Tic disorders. A population-based cohort study. JAMA Psychiatry. 2015;72:787–93.
Browne HA, Hansen SN, Buxbaum JD, Gair SL, Nissen JB, Nikolajsen KH, et al. Familial clustering of tic disorders and obsessive-compulsive disorder. JAMA Psychiatry. 2015;72:359–66.
Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51.
Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18:721–8.
Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, et al. Interrogating the genetic determinants of Tourette’s syndrome and other Tic disorders through genome-wide association studies. Am J Psychiatry. 2019;176:217–27.
Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 2013;9:e1003864.
Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362:1901–8.
Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90.
Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–20.
O’Roak BJ, Morgan TM, Fishman DO, Saus E, Alonso P, Gratacos M, et al. Additional support for the association of SLITRK1 var321 and Tourette syndrome. Mol Psychiatry. 2010;15:447–50.
Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9.
Willsey AJ, Fernandez TV, Yu D, King RA, Dietrich A, Xing J, et al. De novo coding variants are strongly associated with Tourette disorder. Neuron. 2017;94:486–99.e489.
Sundaram SK, Huq AM, Wilson BJ, Chugani HT. Tourette syndrome is associated with recurrent exonic copy number variants. Neurology. 2010;74:1583–90.
Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–8.
Robertson MM, Shelley BP, Dalwai S, Brewer C, Critchley HD. A patient with both Gilles de la Tourette’s syndrome and chromosome 22q11 deletion syndrome: clue to the genetics of Gilles de la Tourette’s syndrome? J Psychosom Res. 2006;61:365–8.
Huang AY, Yu D, Davis LK, Sul JH, Tsetsos F, Ramensky V, et al. Rare copy number variants in NRXN1 and CNTN6 increase risk for Tourette syndrome. Neuron. 2017;94:1101–11.e7.
Ruzzo EK, Perez-Cano L, Jung JY, Wang LK, Kashef-Haghighi D, Hartl C, et al. Inherited and de novo genetic risk for autism impacts shared networks. Cell. 2019;178:850–66.e826.
Andlauer TFM, Guzman-Parra J, Streit F, Strohmaier J, Gonzalez MJ, Gil Flores S, et al. Bipolar multiplex families have an increased burden of common risk variants for psychiatric disorders. Mol Psychiatry. 2019;26:1286–98.
Szatkiewicz J, Crowley JJ, Adolfsson AN, Aberg KA, Alaerts M, Genovese G, et al. The genomics of major psychiatric disorders in a large pedigree from Northern Sweden. Transl Psychiatry. 2019;9:60.
de Jong S, Diniz MJA, Saloma A, Gadelha A, Santoro ML, Ota VK, et al. Applying polygenic risk scoring for psychiatric disorders to a large family with bipolar disorder and major depressive disorder. Commun Biol. 2018;1:163.
Robertson MM, Gourdie A. Familial Tourette’s syndrome in a large British pedigree. Associated psychopathology, severity, and potential for linkage analysis. Br J Psychiatry. 1990;156:515–21.
Curtis D, Robertson MM, Gurling HM. Autosomal dominant gene transmission in a large kindred with Gilles de la Tourette syndrome. Br J Psychiatry. 1992;160:845–9.
Brett PM, Le Bourdelles B, See CG, Whiting PJ, Attwood J, Woodward K, et al. Genomic cloning and localization by FISH and linkage analysis of the human gene encoding the primary subunit NMDAR1 (GRIN1) of the NMDA receptor channel. Ann Hum Genet. 1994;58:95–100.
Brett PM, Curtis D, Robertson MM, Gurling HM. Exclusion of the 5-HT1A serotonin neuroreceptor and tryptophan oxygenase genes in a large British kindred multiply affected with Tourette’s syndrome, chronic motor tics, and obsessive-compulsive behavior. Am J Psychiatry. 1995;152:437–40.
Brett PM, Curtis D, Robertson MM, Gurling HM. The genetic susceptibility to Gilles de la Tourette syndrome in a large multiple affected British kindred: linkage analysis excludes a role for the genes coding for dopamine D1, D2, D3, D4, D5 receptors, dopamine beta hydroxylase, tyrosinase, and tyrosine hydroxylase. Biol Psychiatry. 1995;37:533–40.
Brett PM, Curtis D, Robertson MM, Dahlitz M, Gurling HM. Linkage analysis and exclusion of regions of chromosomes 3 and 8 in Gilles de la Tourette syndrome following the identification of a balanced reciprocal translocation 46 XY, t(3:8)(p21.3 q24.1) in a case of Tourette syndrome. Psychiatr Genet. 1996;6:99–105.
Brett PM, Curtis D, Robertson MM, Gurling HM. Neuroreceptor subunit genes and the genetic susceptibility to Gilles de la Tourette syndrome. Biol Psychiatry. 1997;42:941–7.
Curtis D, Brett P, Dearlove AM, McQuillin A, Kalsi G, Robertson MM, et al. Genome scan of Tourette syndrome in a single large pedigree shows some support for linkage to regions of chromosomes 5, 10 and 13. Psychiatr Genet. 2004;14:83–7.
Robertson MM, Cavanna AE. The Gilles de la Tourette syndrome: a principal component factor analytic study of a large pedigree. Psychiatr Genet. 2007;17:143–52.
Kendall KM, Rees E, Bracher-Smith M, Legge S, Riglin L, Zammit S, et al. Association of rare copy number variants with risk of depression. JAMA Psychiatry. 2019:76:818–25.
Tang H, Kirkness EF, Lippert C, Biggs WH, Fabani M, Guzman E, et al. Profiling of short-tandem-repeat disease alleles in 12,632 human whole genomes. Am J Hum Genet. 2017;101:700–15.
di Iulio J, Bartha I, Wong EHM, Yu HC, Lavrenko V, Yang D, et al. The human noncoding genome defined by genetic diversity. Nat Genet. 2018;50:333–7.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Sherman T, Fu J, Scharpf RB, Bureau A, Ruczinski I. Detection of rare disease variants in extended pedigrees using RVS. Bioinformatics. 2019;35:2509–11.
International Obsessive Compulsive Disorder Foundation Genetics Collaborative and OCD Collaborative Genetics Association Studies. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23:1181–8.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75.
Chen H, Wang C, Conomos MP, Stilp AM, Li Z, Sofer T, et al. Control for population structure and relatedness for binary traits in genetic association studies via logistic mixed models. Am J Hum Genet. 2016;98:653–66.
Collins AL, Kim Y, Szatkiewicz JP, Bloom RJ, Hilliard CE, Quackenbush CR, et al. Identifying bipolar disorder susceptibility loci in a densely affected pedigree. Mol Psychiatry. 2013;18:1245–6.
Diniz MJA, Rodrigues ACS, Gadelha A, Alsabban S, Guindalini C, Paya-Cano J, et al. Whole genome linkage analysis in a large Brazilian multigenerational family reveals distinct linkage signals for bipolar disorder and depression. bioRxiv. 2017. https://doi.org/10.1101/106260.
Nordsletten AE, Larsson H, Crowley JJ, Almqvist C, Lichtenstein P, Mataix-Cols D. Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry. 2016;73:354–61.
Kurlan R, Eapen V, Stern J, McDermott MP, Robertson MM. Bilineal transmission in Tourette’s syndrome families. Neurology. 1994;44:2336–42.
Acknowledgements
Genotyping and sequencing was performed by the SNP&SEQ Technology Platform in Uppsala, Sweden. The facility is part of the National Genomics Infrastructure (NGI) Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. Dr. Crowley was supported by NIH grants R01MH105500 and R01MH110427 and the Foundation of Hope. We finally wish to thank once again, the pedigree members, albeit years ago, for their extraordinary help and cooperation with the interviews, examnation, and venepunctures.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
DMC reports receiving personal fees for editorial tasks (Elsevier) and contributing articles (UpToDate, Inc), both outside the submitted work. CAM has received fees from W.W. Norton and Co. for a book on hoarding disorder, also outside the submitted work. The remaining authors report no biomedical financial interests or potential competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Halvorsen, M., Szatkiewicz, J., Mudgal, P. et al. Elevated common variant genetic risk for tourette syndrome in a densely-affected pedigree. Mol Psychiatry 26, 7522–7529 (2021). https://doi.org/10.1038/s41380-021-01277-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01277-w