Abstract
Ancillary studies facilitate accurate diagnosis of morphologically challenging mesothelial proliferations. The current diagnostic algorithm proceeds from BAP1 immunohistochemistry to CDKN2A fluorescence in situ hybridization. While MTAP immunohistochemistry has recently shown promise as a surrogate for CDKN2A fluorescence in situ hybridization, it has been examined in only a few single-institution studies. Furthermore, there are no published reports on interobserver agreement or interlaboratory reproducibility for MTAP immunohistochemistry. We performed MTAP immunohistochemistry on 20 benign mesothelial lesions and 99 malignant mesotheliomas from five mesothelioma centers in four countries, and each MTAP stain was independently interpreted by four pathologists. CDKN2A fluorescence in situ hybridization data were available for a subset of cases, and a subset of cases was subjected in MTAP immunohistochemistry in multiple laboratories to assess interlaboratory reproducibility. Interobserver agreement in MTAP immunostain interpretation was excellent for all mesothelial lesions (kappa: 0.85) and for malignant mesothelioma cases only (kappa: 0.82). Interlaboratory reproducibility was also excellent (kappa values for paired protocols: 0.77–0.89). MTAP loss by immunohistochemistry was 78% sensitive and 96% specific for CDKN2A homozygous deletion. MTAP immunohistochemistry is a reliable surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant mesothelioma. Interobserver agreement is excellent for interpretation of MTAP staining, and protocols performed in different laboratories yield concordant MTAP staining results. Rare cases with immunohistochemical MTAP loss may retain normal CDKN2A copy number, and the MTAP staining results should be correlated with clinicopathologic findings and other ancillary studies.
Similar content being viewed by others
Introduction
Malignant mesothelioma is an aggressive malignancy arising from the serosal lining of body cavities. Approximately 85–90% of mesotheliomas arise from the pleura, with most of the remainder arising from the peritoneum [1]. The histomorphologic gold standard for diagnosing malignancy in a mesothelial proliferation is invasion into adjacent tissues; however, histomorphology alone may be insufficient for definitive diagnosis of mesothelioma, particularly in biopsies or in cell-block preparations of pleural or peritoneal effusions [2,3,4,5]. Furthermore, recent reports provide evidence for an in situ form of mesothelioma, which may present with recurrent effusion and undergo multiple biopsies, showing suspicious cytologic features without invasion. In such cases, ancillary techniques are required to demonstrate the malignant biologic potential of the lesion [6].
Ancillary tests can help distinguish a florid-reactive mesothelial proliferation from mesothelioma in cases with ambiguous histomorphology and can assist in defining the malignant elements of a biphasic mesothelioma [7]. Immunohistochemical loss of BRCA1-associated protein 1 (BAP1) is highly specific for distinguishing mesothelioma from both benign mesothelial proliferations and pulmonary carcinoma [5, 8,9,10,11,12,13,14,15,16,17]. However, BAP1 loss is observed in just 55% of pleural mesotheliomas overall, including as few as 7% of sarcomatoid mesotheliomas [17]. The CDKN2A locus on chromosome 9p21 encodes p16 protein, and CDKN2A homozygous deletion by fluorescence in situ hybridization (FISH) is diagnostic of malignancy in a mesothelial proliferation. Thus, CDKN2A FISH can be performed in challenging mesothelial lesions with retained BAP1, although CDKN2A FISH reportedly occurs in only 52–68% of mesotheliomas [5, 10, 18,19,20,21,22]. Longer turnaround times, greater expense, and lower availability of FISH compared with immunohistochemistry have prompted interest in a reliable immunohistochemical surrogate for CDKN2A FISH, but immunohistochemical loss of p16 or related proteins p14 and p15 is not specific for CDKN2A homozygous deletion [14, 19].
The MTAP gene is located adjacent to CDKN2A on chromosome 9p21. Its protein product, methylthioadenosine phosphorylase (MTAP), is critical for adenosine monophosphate and methionine salvage, and the potential for therapies exploiting synthetic lethality in MTAP-deficient tumors has been discussed [23,24,25]. Early FISH studies showed MTAP co-deletion in up to 90% of pleural and peritoneal mesotheliomas with CDKN2A homozygous deletion [18, 19, 23]. However, MTAP FISH has not been adopted for routine clinical diagnosis.
One early study utilizing a polyclonal anti-MTAP primary antibody showed disappointing specificity of MTAP immunohistochemistry for diagnosis of mesothelioma [26]. However, multiple more recent studies using a monoclonal anti-MTAP primary antibody report excellent specificity and acceptable sensitivity of MTAP immunohistochemistry for detection of CDKN2A homozygous deletion and diagnosis of mesothelioma [10, 14, 27]. Subsequent studies also showed that MTAP immunohistochemistry could be reliably applied to cell-block preparations [2, 27] and in the differential diagnosis of sarcomatoid mesothelial lesions [27, 28]. These results have substantially increased enthusiasm for clinical uptake of MTAP immunohistochemistry, but multi-institutional studies of the reliability and reproducibility of MTAP immunostaining are not yet published. Here, we report an international investigation of interobserver agreement and interlaboratory reproducibility of MTAP immunohistochemical staining. Comparison of MTAP immunohistochemistry to available CDKN2A FISH data in a subset of cases offers further insights on its reliability as a surrogate marker for FISH in mesothelioma diagnosis.
Methods
Cohort selection
The study was performed with approval from institutional review boards at the collaborating institutions (University of Chicago #17–53). Cases were contributed from five mesothelioma referral centers in four countries (Japan, Canada, France, and the United States). The collaborators contributed to the cohort, as follows:
-
AC and KB contributed a tissue microarray of 53 cases, including 20 reactive mesothelial proliferations and 33 mesotheliomas. Nineteen of 20 reactive mesothelial proliferations and 32/33 mesotheliomas were represented in the tissue microarray by two 1-mm cores. One mesothelioma and one reactive mesothelial proliferation were represented by a single 1-mm core. MTAP immunohistochemistry and CDKN2A FISH data on these cases have been previously published [10].
-
KH contributed 11 mesotheliomas on whole-slide sections (2 resections, 9 biopsies). KN contributed 25 mesotheliomas on whole-slide sections (13 resections, 4 biopsies, 8 cell blocks—MTAP immunohistochemistry and CDKN2A FISH data on these cases previously published [14]). FG-S and NLS contributed seven mesotheliomas on whole-slide sections (three resections, four biopsies). DBC, JS, and ANH contributed 23 mesotheliomas on whole-slide sections (22 resections, 1 biopsy).
Hematoxylin and eosin (H&E)-stained sections from each case were scored centrally (DBC) for lymphohistiocytic infiltrate and spindled morphology.
MTAP immunohistochemistry protocols
Four of five collaborating institutions had a validated in-house MTAP immunohistochemistry protocol: Fukuoka University, Tokyo Women’s Medical University, the University of Chicago, and Vancouver General Hospital (Table 1). Protocols for MTAP immunohistochemistry from Vancouver General Hospital [10] and Fukuoka University [28] have been previously published. Briefly, the Vancouver General Hospital and Fukuoka University protocols both used the mouse monoclonal anti-MTAP clone 2G4 (Abnova, Taipei, Taiwan) at 1:100 dilution. For this study, Fukuoka University employed two additional staining protocols using the same antibody: a “moderately strong” condition (1:50 primary antibody dilution) and a “strong” condition (1:50 primary antibody dilution, incubated with a FLEX mouse linker enhancer [Dako, Agilent, Santa Clara, California]).
The Tokyo Women’s Medical University protocol used the mouse monoclonal anti-MTAP clone DA2 (Abnova) at 1:200 dilution following antigen retrieval by 20 min of heat incubation in Bond epitope retrieval solution 2 (Leica Biosystems, AR9640), with staining was performed on a Leica Bond Max automatic stainer. The University of Chicago protocol used the mouse monoclonal anti-MTAP clone 42-T (Santa Cruz, sc-10078) at 1:100 dilution, following antigen retrieval by 20 min of heat incubation in Bond epitope retrieval solution 2 (Leica Biosystems), with staining performed on a Leica Bond RX automatic stainer and antigen–antibody binding detected with Bond polymer refine detection (Leica Biosystems, DS9800), with hematoxylin counterstaining.
Evaluation of interobserver agreement of MTAP immunohistochemistry
Four pathologists independently scored each case in the cohort for MTAP loss or retention, based on their own practice standards, as informed by published literature on MTAP immunohistochemistry. Generally, MTAP retention is characterized by cytoplasmic staining in tumor cells, and MTAP loss by absence of cytoplasmic staining in tumor cells in the presence of a positive internal control [10]. Nuclear MTAP staining is reported in a subset of cases but is inconsistent, and absence of nuclear MTAP with retention of cytoplasmic MTAP staining is regarded as MTAP retention [10, 14].
All 119 cases were independently evaluated by DBC, JS, and ANH. The contributing pathologist from each collaborating institution served as the fourth evaluator for each case, with the exception of the cases from MESOPATH (fourth evaluator: TK) and the University of Chicago (fourth evaluator: KN). Cases were excluded from further analysis if they had insufficient tumor tissue for evaluation by all four scoring pathologists or if the positive internal control failed.
Any case called “MTAP retained” by at least one scorer was reviewed by two pathologists (DBC, JS), and the MTAP staining was termed “faint” if there was consensus that tumor cell cytoplasmic staining was incontrovertibly fainter than internal control staining.
Evaluation of interlaboratory reproducibility of MTAP immunohistochemistry
A subset of cases was stained in two different pathology laboratories, using the individual lab’s MTAP immunohistochemistry protocol. Twenty-three cases from the University of Chicago were also stained at Fukuoka University (using standard, moderately strong, and strong staining conditions; see above); 25 cases from Fukuoka University were also stained at the University of Chicago; and the tissue microarray from Vancouver General Hospital (including 20 reactive mesothelial benign controls and 33 malignant mesotheliomas) was also stained at the University of Chicago.
All slides stained in two laboratories were evaluated independently by three pathologists (DBC, JS, and ANH). To blind the scoring of the two slide sets, a 2-month “washout” was allowed between scoring of slides from the first lab and scoring of slides from the second lab. During this 2-month period, no intervening factors significantly altered, enhanced, or reduced the observers’ evaluation of MTAP immunostains.
CDKN2A fluorescence in situ hybridization
Protocols for CDKN2A FISH from Vancouver General Hospital [10], Fukuoka University [28], Tokyo Women’s Medical University [7], and the University of Chicago [16] have already been published. Briefly, the Vancouver General Hospital and Tokyo Women’s Medical University protocols used a CDKN2A probe and CEP9 probe from Abbott Molecular (Des Plaines, IL, USA). The Fukuoka University protocol used a 190 kb Vysis LSI p16/CEP 9 probe from Abbott Japan (Tokyo, Japan). The University of Chicago protocol used a custom CDKN2A probe generated with a bacterial artificial clone (RP11–14912) containing CDKN2A-specific sequences.
Statistical analyses
Interobserver agreement between the four pathologists scoring MTAP immunohistochemistry in each case was assessed using Fleiss’s free marginal kappa. Association of interobserver disagreement with binary categorical variables was assessed by chi-squared testing. Interlaboratory reproducibility was evaluated by assessing intraobserver agreement for each of the three individual observers and for the three observers’ pooled data using Cohen’s kappa. Kappa values were grouped using the system proposed by Fleiss, with kappa < 0.40 considered “poor,” 0.40–0.75 considered “fair,” and > 0.75 considered “excellent” [29]. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Interobserver agreement in MTAP immunohistochemistry scoring
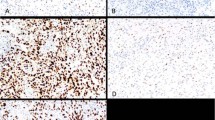
Of 119 MTAP-stained cases (20 reactive mesothelial proliferations and 99 mesotheliomas), all four observers were able to score MTAP expression in 107 cases (18 reactive mesothelial proliferations and 89 mesotheliomas) (Table 2). Interobserver agreement in MTAP scoring was excellent, with concordant MTAP scoring by all four observers (i.e., interobserver consensus) in 92 of 107 total cases (86.0%; Fleiss’s free marginal kappa for all cases: 0.85 [95% CI, 0.79–0.92]) and in 74 of 89 mesotheliomas (83.1%; Fleiss’s free marginal kappa for mesotheliomas only: 0.82 [95% CI, 0.74–0.90]). Of the 74 mesotheliomas with interobserver consensus, 40 (54.1%) retained MTAP (Fig. 1a, b) and 34 (45.9%) lost MTAP (Fig. 1c, d).
a, b A pleural epithelioid malignant mesothelioma with retained cytoplasmic MTAP staining. c, d A different pleural epithelioid malignant mesothelioma shows loss of cytoplasmic MTAP staining. Stromal cells and tumor-infiltrating inflammatory cells provide a positive internal control. a, c Hematoxylin and eosin. b, d MTAP immunohistochemistry with diaminobenzidine chromogen. Original magnification for all photomicrographs, ×100
Among mesotheliomas, interobserver disagreement on MTAP scoring was significantly more likely in cases with an intratumoral lymphohistiocytic infiltrate identified on H&E-stained slides (p = 0.009) and in cases with faint MTAP staining (p < 0.0001) (Fig. 2). Furthermore, faint MTAP was significantly associated with lymphohistiocytic infiltrate (p < 0.0001). Interobserver disagreement on MTAP scoring was not related to tumor morphology (spindled vs epithelioid; p = 0.80); to whether the evaluated tissue was from a biopsy, cell block, or resection (p = 0.46); or to whether the evaluated tissue was in a tissue microarray or whole-section slide (p = 0.10).
A pleural epithelioid malignant mesothelioma with lymphohistiocytic infiltrate (a) and faint MTAP staining (b). Tumoral cytoplasmic staining is present but substantially weaker than the positive internal control. All 11 mesotheliomas with faint MTAP in this study showed CDKN2A homozygous deletion by FISH. a Hematoxylin and eosin. b MTAP immunohistochemistry with diaminobenzidine chromogen. Original magnification for all photomicrographs, ×100
Concordance of MTAP immunohistochemistry with CDKN2A FISH
Of 69 mesotheliomas with CDKN2A FISH results, 43 (62%) showed CDKN2A homozygous deletion, and 26 (38%) showed normal CDKN2A copy number. Fifty-six mesotheliomas had both FISH data and interobserver consensus on MTAP scoring. MTAP loss was 78% sensitive and 96% specific for CDKN2A homozygous deletion (Table 3A). Eleven of 11 cases with faint MTAP staining showed homozygous deletion of CDKN2A.
CDKN2A FISH data were available in an additional 13 cases with interobserver disagreement on MTAP scoring. Of these 13 cases, 11 (85%) showed CDKN2A homozygous deletion by FISH, and 2 (15%) showed normal CDKN2A copy number. Although not reaching statistical significance, there is a strong trend toward greater interobserver disagreement on MTAP scoring in cases with CDKN2A homozygous deletion, compared with cases with normal CDKN2A copy number (p = 0.07).
Evaluation of cell block and biopsy specimens
Among biopsy specimens, there was interobserver consensus on MTAP scoring in 20/24 cases. Seventeen biopsies had both FISH data and interobserver consensus on MTAP scoring, with MTAP loss 67% sensitive and 100% specific for CKDN2A deletion among biopsy specimens (Table 3B). Among cell-block specimens, there was interobserver consensus on MTAP scoring in 7/8 cases. Seven cell block specimens had both FISH data and interobserver consensus on MTAP scoring, with MTAP loss 75% sensitive and 100% specific for CKDN2A deletion among biopsy specimens (Table 3C). Sensitivity and specificity of MTAP immunohistochemistry for detection of CDKN2A homozygous deletion, as well as interobserver agreement on MTAP scoring, did not differ significantly between cell block, biopsy, and resection specimens.
Interlaboratory reproducibility of MTAP immunohistochemistry
The cohort for interlaboratory reproducibility calculations included 81 mesotheliomas and 18 reactive mesothelial proliferations. Interlaboratory reproducibility of MTAP immunohistochemistry scoring was excellent, with Cohen kappa of 0.77 (95% CI, 0.69–0.85) for comparison of Vancouver General Hospital and University of Chicago protocols, and Cohen kappa of 0.89 (95% CI, 0.81–0.96) for comparison of the University of Chicago protocol with the standard and moderately strong Fukuoka University protocols (Fig. 3). Conversely, concordance between the University of Chicago protocol and the Fukuoka University “strong” protocol was poor, with Cohen kappa of 0.39 (95% CI, 0.17–0.62).
A pleural epithelioid malignant mesothelioma (a) with interobserver consensus for MTAP loss by the Fukuoka University standard protocol (b) (anti-MTAP clone 2G4, 1:100) and consensus for MTAP retention by the University of Chicago protocol (c) (anti-MTAP clone 42-T, 1:100). a Hematoxylin and eosin. b, c MTAP immunohistochemistry with diaminobenzidine chromogen. Original magnification for all photomicrographs, ×100
Unusual patterns of MTAP immunohistochemical staining
Four mesotheliomas showed heterogeneous MTAP expression in tumor cells. Three cases showed discrete areas of MTAP retention and loss, consistent with subclonal MTAP loss (Fig. 4a). The fourth case showed strong nuclear staining in a subset of tumor cells, with complete absence of cytoplasmic staining (Fig. 4b). All four of these cases showed CDKN2A homozygous deletion by FISH, although it is not certain whether the cells evaluated by FISH included those with MTAP retention or loss by immunohistochemistry, or a combination of these two populations.
Discussion
This multi-institutional study of MTAP immunohistochemistry demonstrates excellent interobserver agreement in MTAP scoring and excellent reproducibility of MTAP staining in laboratories using different immunohistochemistry protocols. The study also provides additional data points on concordance of MTAP immunohistochemistry with gold-standard CDKN2A FISH.
Malignant mesothelioma is an aggressive neoplasm with high morbidity and mortality, and treatment of mesothelioma includes surgery, chemotherapy, or both [30]. Given the gravity of this diagnosis, ancillary studies for diagnosis of mesothelioma must demonstrate a high degree of specificity. Furthermore, because mesothelioma patients are often referred to tertiary centers for management and because second opinions are frequently solicited for this diagnosis, ancillary studies used in mesothelioma diagnosis should have a high degree of interpretive agreement between pathologists reviewing the same slides, and a high degree of reproducibility between laboratories performing studies on the same tissue specimens.
Multiple studies published in recent years have provided convincing evidence for high specificity of MTAP immunohistochemistry for detection of CDKN2A homozygous deletion [10, 14, 27], which is itself considered a highly specific gold-standard marker of malignancy in mesothelial lesions. Our data address multiple questions not examined in previous studies. First, we show excellent agreement in interpretation of MTAP immunostains among pathologists at four institutions. This finding offers pathologists, clinicians, and patients alike a high degree of confidence in reported MTAP immunohistochemistry results. Importantly, our data show excellent interobserver agreement for MTAP scoring in cell blocks and biopsies, as well as in resection specimens, providing additional support for earlier reports of the accuracy of MTAP immunohistochemistry in small diagnostic specimens [2].
Our data highlight common areas of uncertainty in scoring MTAP immunohistochemistry. In tumors with a lymphohistiocytic infiltrate, pathologists were more likely to disagree on the tumor’s MTAP status. Because cytoplasmic MTAP protein is expressed in normal cells, large tumor-infiltrating histiocytes appear to mimic MTAP-positive epithelioid tumor cells in some cases, particularly when the infiltrate is brisk or tumor cells are sparse. Similarly, in sarcomatoid mesothelioma, histiocytic processes can extend between and around poorly demarcated tumor cells, which may make it difficult to assess whether positive staining is present within or rather adjacent to tumor cells. Awareness of this pitfall, together with evaluation of MTAP immunostains at high magnification in cases with lymphohistiocytic infiltrate, can likely facilitate accurate interpretation in most cases. Application of a double immunostain for MTAP and a nuclear mesothelial marker, such as WT-1, could also be considered in future studies.
Interobserver disagreement on MTAP scoring was also significantly more likely in cases with faint cytoplasmic MTAP, defined as tumoral cytoplasmic staining that is overtly weaker than the cytoplasmic staining in the positive internal control. Hida et al. considered faint staining as evidence for CDKN2A homozygous deletion [14]. Our data support this interpretation: All 11 cases we observed with faint MTAP staining in tumor cytoplasm showed CDKN2A homozygous deletion by FISH. Importantly, such interpretation of faint tumoral MTAP staining relies on a robust positive internal control, to prevent overcalling of CDKN2A deletion in cases with artefactual loss of MTAP due to poor fixation or failure of staining. Interestingly, we found an association between tumoral lymphohistiocytic infiltrates and faint MTAP, suggesting that, in some cases, intercalating histiocytic processes may be responsible for the appearance of faint MTAP staining in tumor cells. Given the element of subjectivity in comparing tumor and internal control staining, there will likely be some cases that fall into a grey zone. In such cases, consultation with other pathologists and correlation with other studies (including CDKN2A FISH, if needed) is advisable.
Our findings validate the work of Berg [10] and Hida [14] regarding the sensitivity and specificity of MTAP immunohistochemistry for detecting CDKN2A homozygous deletion. First, the excellent agreement of our multi-institutional cohort of pathologists in MTAP scoring in a subset of previously reported mesotheliomas from these investigators further substantiates their previously published work [10, 14]. Second, this study includes 24 additional mesotheliomas, which further validate a sensitivity and specificity of ~80% and nearly 100%, respectively, for detection of CDKN2A homozygous deletion with MTAP immunohistochemistry. Others have additionally reported that a panel including BAP1 and MTAP immunostains achieves up to 90% sensitivity and 100% specificity for diagnosis of mesothelioma [10, 14, 27].
Importantly, however, our data verify the finding of Berg et al. [10] that rare mesothelial proliferations with normal CDKN2A copy number show MTAP loss, as seen in one case in this cohort (which showed interobserver consensus for MTAP loss and adequate internal control staining). This single case with “false positive” MTAP loss raises the possibility that a reactive mesothelial proliferation with MTAP loss, but normal CDKN2A copy number could be misdiagnosed as malignant. However, in this study, the case in question was nonetheless a mesothelioma on the basis of histomorphology and clinical behavior, and benign mesothelial proliferations with MTAP loss have not yet been reported in studies utilizing monoclonal anti-MTAP primary antibodies. The biological basis of these rare cases with MTAP loss by normal CDKN2A copy number remains unclear, although it may represent epigenetic silencing of the MTAP gene [31] or selective deletion of the MTAP locus, without CDKN2A alterations.
This is the first study of MTAP immunohistochemistry to directly compare the results of staining protocols performed in different laboratories. We found excellent concordance between the pairs of laboratories studied, including those using different anti-MTAP primary antibody clones (2G4 vs 42-T) and dilutions (1:100 vs 1:50). There were, however, rare cases in which all independent observers scored a case as MTAP-retained based on staining from one institution, but MTAP-lost based on staining from another institution (see Fig. 3). These findings indicate that the MTAP immunostain results obtained at different institutions can be reliably compared, but that differences in MTAP immunohistochemistry protocols may account for discordant interpretations in some cases.
Our comparisons between laboratories also indicate that overly strong staining conditions (namely, a protocol using a 1:50 dilution of primary antibody and an enhancing linker to repeat staining on 23 mesotheliomas) can produce nonspecific cytoplasmic staining in cases with consensus for MTAP loss on standard staining conditions, thus substantially reducing the assay’s sensitivity for detecting CDKN2A homozygous deletion.
The significance of subclonal heterogeneity of MTAP staining within a tumor remains unclear. In some cases, it may represent reversible epigenetic silencing of MTAP through promoter hypermethylation [31], whereas in other cases it may reflect emergence of a subclone with CDKN2A deletion, which may in turn signify greater malignant potential. All three cases in this cohort with subclonal MTAP loss showed CDKN2A homozygous deletion by FISH. This supports the assertion that at least a subpopulation in these tumors harbors a 9p21 deletion [10]; however, because FISH results in our study were obtained from records, it is unclear whether the cells evaluated by FISH correspond to those with retained or lost MTAP. Of course, distinct subclonal staining should be distinguished from variable or patchy staining, which more likely reflects artifact secondary to poor fixation, suboptimal stain performance, or other pre-analytical factors. We also observed one pleomorphic epithelioid mesothelioma with complete absence of cytoplasmic staining, but strong, patchy nuclear staining. This case also showed CDKN2A homozygous deletion by FISH. The biology of such nuclear-only staining is unclear, although correlation of MTAP immunohistochemistry with FISH data in this case appears to support the diagnostic primacy of cytoplasmic MTAP staining. Additional studies on mesothelioma tumorigenesis would help clarify both the biological and the clinical significance of heterogeneous MTAP staining. Overall, these unusual MTAP patterns appear to be rare in mesothelioma, occurring in only 4/102 (4%) of cases in this cohort.
Certain limitations of this study warrant discussion and consideration in subsequent studies. First, our examination of interobserver agreement involved pathologists with expertize in mesothelioma, so their interpretations of MTAP immunostains may not entirely reflect the community of surgical pathologists. However, because this is a relatively new marker not yet in widespread clinical use, MTAP immunohistochemistry is currently generally available only at such mesothelioma centers, and even mesothelioma specialists have relatively limited experience with it. We believe that the high degree of interobserver agreement in such a new marker indicates that MTAP immunohistochemistry can be widely and reliably employed as a diagnostic marker for mesothelioma, particularly as studies such as ours refine the interpretation recommendations for challenging cases, and as the pathology community gains experience with its use and interpretation.
Due to logistical limitations on transfer of tissue, we were not able to compare staining performed at Tokyo Women’s Medical University to staining protocols at other institutions, which limits our ability to comment on reproducibility of staining at 1:200 primary antibody dilution or using the DA2 anti-MTAP clone. Given the preponderance of data supporting the accuracy and reproducibility of MTAP staining and MTAP scoring at 1:100 primary antibody dilution, this may be a good starting dilution for validation of MTAP immunostaining in additional laboratories.
In conclusion, immunohistochemical staining for MTAP is a surrogate marker for CDKN2A FISH that shows excellent interobserver agreement and interlaboratory reproducibility. Loss of MTAP shows approximately 80% sensitivity and nearly 100% specificity for detecting CDKN2A homozygous deletion. Additional investigation of rare cases with MTAP loss but normal CDKN2A copy number is necessary to better understand the biology and clinical significance of this finding. However, substantial evidence indicates that MTAP immunohistochemistry is a robust and accurate diagnostic marker, and we advocate its incorporation into the routine diagnostic workup for malignant mesothelioma.
References
Beebe-Dimmer JL, Fryzek JP, Yee CL, Dalvi TB, Garabrant DH, Schwartz AG, et al. Mesothelioma in the United States: a surveillance, epidemiology, and end results (SEER)-Medicare investigation of treatment patterns and overall survival. Clin Epidemiol. 2016;8:743–50.
Kinoshita Y, Hida T, Hamasaki M, Matsumoto S, Sato A, Tsujimura T, et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol. 2018;126:54–63.
Schappa JT, Foutz CA, Olson EJ, Armien AG, Ward C, Sharkey LC. What is your diagnosis? Bicavitary effusion in a horse. Vet Clin Pathol. 2017;46:189–90.
Paintal A. The evolving role of effusion cytology in the diagnosis of malignant mesothelioma. Cytopathology. 2015;26:137–8.
Hwang HC, Sheffield BS, Rodriguez S, Thompson K, Tse CH, Gown AM, et al. Utility of BAP1 immunohistochemistry andp16 (cdkn2a) fish in the diagnosis of malignant mesothelioma in effusion cytology specimens. Am J Surg Pathol. 2016;40:120–6.
Churg A, Hwang H, Tan L, Qing G, Taher A, Tong A, et al. Malignant mesothelioma in situ. Histopathology. 2018;72:1033–8.
Wu D, Hiroshima K, Yusa T, Ozaki D, Koh E, Sekine Y, et al. Usefulness of p16/CDKN2A fluorescence in situ hybridization and BAP1 immunohistochemistry for the diagnosis of biphasic mesothelioma. Ann Diagn Pathol. 2017;26:31–7.
Andrici J, Jung J, Sheen A, D’Urso L, Sioson L, Pickett J, et al. Loss of BAP1 expression is very rare in peritoneal and gynecologic serous adenocarcinomas and can be useful in the differential diagnosis with abdominal mesothelioma. Hum Pathol. 2016;51:9–15.
Andrici J, Parkhill TR, Jung J, Wardell KL, Verdonk B, Singh A, et al. Loss of expression of BAP1 is very rare in non-small cell lung carcinoma. Pathology. 2016;48:336–40.
Berg KB, Dacic S, Miller C, Cheung S, Churg A. Utility of methylthioadenosine phosphorylase compared with BAP1 immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch Pathol Lab Med. 2018;142:1549–53.
Carbone M, Shimizu D, Napolitano A, Tanji M, Pass HI, Yang H, et al. Positive nuclear BAP1 immunostaining helps differentiate non-small cell lung carcinomas from malignant mesothelioma. Oncotarget. 2016;7:59314–21.
Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28:1043–57.
Cozzi I, Oprescu FA, Rullo E, Ascoli V. Loss of BRCA1-associated protein 1 (BAP1) expression is useful in diagnostic cytopathology of malignant mesothelioma in effusions. Diagn Cytopathol. 2018;46:9–14.
Hida T, Hamasaki M, Matsumoto S, Sato A, Tsujimura T, Kawahara K, et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer. 2017;104:98–105.
Leblay N, Lepretre F, Le Stang N, Gautier-Stein A, Villeneuve L, Isaac S, et al. BAP1 is altered by copy number loss, mutation, and/or loss of protein expression in more than 70% of malignant peritoneal mesotheliomas. J Thorac Oncol. 2017;12:724–33.
McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol. 2015;46:1670–8.
Wang LM, Shi ZW, Wang JL, Lv Z, Du FB, Yang QB, et al. Diagnostic accuracy of BRCA1-associated protein 1 in malignant mesothelioma: a meta-analysis. Oncotarget. 2017;8:68863–72.
Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–13.
Krasinskas AM, Bartlett DL, Cieply K, Dacic S. CDKN2A and MTAP deletions in peritoneal mesotheliomas are correlated with loss of p16 protein expression and poor survival. Mod Pathol. 2010;23:531–8.
Prins JB, Williamson KA, Kamp MM, Van Hezik EJ, Van der Kwast TH, Hagemeijer A, et al. The gene for the cyclin-dependent-kinase-4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. Int J Cancer. 1998;75:649–53.
Sheffield BS, Hwang HC, Lee AF, Thompson K, Rodriguez S, Tse CH, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol. 2015;39:977–82.
Cheng JQ, Jhanwar SC, Lu YY, Testa JR. Homozygous deletions within 9p21-p22 identify a small critical region of chromosomal loss in human malignant mesotheliomas. Cancer Res. 1993;53:4761–3.
Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer. 2005;49(Suppl 1):S95–8.
Powell EL, Leoni LM, Canto MI, Forastiere AA, Iocobuzio-Donahue CA, Wang JS, et al. Concordant loss of MTAP and p16/CDKN2A expression in gastroesophageal carcinogenesis: evidence of homozygous deletion in esophageal noninvasive precursor lesions and therapeutic implications. Am J Surg Pathol. 2005;29:1497–504.
Marjon K, Cameron MJ, Quang P, Clasquin MF, Mandley E, Kunii K, et al. MTAP deletions in cancer create vulnerability to targeting of the MAT2A/PRMT5/RIOK1 axis. Cell Rep. 2016;15:574–87.
Zimling ZG, Jorgensen A, Santoni-Rugiu E. The diagnostic value of immunohistochemically detected methylthioadenosine phosphorylase deficiency in malignant pleural mesotheliomas. Histopathology. 2012;60:E96–105.
Churg A, Nabeshima K, Ali G, Bruno R, Fernandez-Cuesta L, Galateau-Salle F. Highlights of the 14th international mesothelioma interest group meeting: pathologic separation of benign from malignant mesothelial proliferations and histologic/molecular analysis of malignant mesothelioma subtypes. Lung Cancer. 2018;124:95–101.
Kinoshita Y, Hamasaki M, Yoshimura M, Matsumoto S, Sato A, Tsujimura T, et al. A combination of MTAP and BAP1 immunohistochemistry is effective for distinguishing sarcomatoid mesothelioma from fibrous pleuritis. Lung Cancer. 2018;125:198–204.
Fleiss J. Statistical methods for rates and proportions. 2 edn. New York, NY: John Wiley; 1981.
Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. NCCN guidelines insights: malignant pleural mesothelioma, version 3.2016. J Natl Compr Canc Netw. 2016;14:825–36.
Behrmann I, Wallner S, Komyod W, Heinrich PC, Schuierer M, Buettner R, et al. Characterization of methylthioadenosin phosphorylase (MTAP) expression in malignant melanoma. Am J Pathol. 2003;163:683–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A portion of this work was presented in March 2019 at the 108th annual meeting of the United States and Canadian Academy of Pathology in National Harbor, Maryland, USA.
Rights and permissions
About this article
Cite this article
Chapel, D.B., Schulte, J.J., Berg, K. et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 33, 245–254 (2020). https://doi.org/10.1038/s41379-019-0310-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0310-0
This article is cited by
-
Expression of fibroblast growth factor receptor 2 (FGFR2) in combined hepatocellular-cholangiocarcinoma and intrahepatic cholangiocarcinoma: clinicopathological study
Virchows Archiv (2024)
-
Loss of methylthioadenosine phosphorylase immunoreactivity correlates with poor prognosis and elevated uptake of 11C-methionine in IDH-mutant astrocytoma
Journal of Neuro-Oncology (2024)
-
Correlation of MTAP immunohistochemical deficiency with CDKN2A homozygous deletion and clinicopathological features in pleomorphic xanthoastrocytoma
Brain Tumor Pathology (2023)
-
Multiomic analysis of malignant pleural mesothelioma identifies molecular axes and specialized tumor profiles driving intertumor heterogeneity
Nature Genetics (2023)
-
Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System
Laboratory Investigation (2022)