Abstract
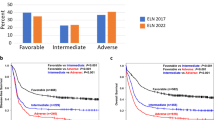
It is vital for physicians and persons with chronic myeloid leukemia (CML) to accurately predict the likelihood of achieving a major molecular response (MMR) and a deep molecular response (DMR; at least MR4) at the start of imatinib-therapy, which could help in decision making of treatment goals and strategies. To answer this question, we interrogated data from 1369 consecutive subjects with chronic phase CML receiving initial imatinib-therapy to identify predictive co-variates. Subjects were randomly-assigned to training (n = 913) and validation (n = 456) datasets. Male sex, higher WBC concentration, lower haemoglobin concentration, higher percentage blood blasts and larger spleen size were significantly-associated with lower cumulative incidences of MMR and MR4 in training dataset. Using Fine-Gray model, we developed the predictive scoring systems for MMR and MR4 which classified subjects into the low-, intermediate- and high-risk cohorts with significantly-different cumulative incidences of MMR and MR4 with good predictive discrimination and accuracy in training and validation cohorts with high area under the receiver-operator characteristic curve (AUROC) values. These data may help physicians decide appropriateness of initial imatinib therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Berman E. How I treat chronic phase chronic myelogenous leukemia. Blood. 2021;139:3138–47.
Jabbour E. Chronic myeloid leukemia: First-line drug of choice. Am J Hematol. 2016;91:59–66.
Oehler VG. First-generation vs second-generation tyrosine kinase inhibitors: which is best at diagnosis of chronic phase chronic myeloid leukemia? Hematol Am Soc Hematol Educ Program. 2020;2020:228–36.
Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121:3818–24.
Castagnetti F, Gugliotta G, Breccia M, Iurlo A, Levato L, Albano F, et al. The BCR-ABL1 transcript type influences response and outcome in Philadelphia chromosome-positive chronic myeloid leukemia patients treated frontline with imatinib. Am J Hematol. 2017;92:797–805.
Ercaliskan A, Eskazan AE. The impact of BCR-ABL1 transcript type on tyrosine kinase inhibitor responses and outcomes in patients with chronic myeloid leukemia. Cancer. 2018;124:3806–18.
Ko PS, Yu YB, Liu YC, Wu YT, Hung MH, Gau JP, et al. Moderate anemia at diagnosis is an independent prognostic marker of the EUTOS, Sokal, and Hasford scores for survival and treatment response in chronic-phase, chronic myeloid leukemia patients with frontline imatinib. Curr Med Res Opin. 2017;33:1737–44.
Nteliopoulos G, Bazeos A, Claudiani S, Gerrard G, Curry E, Szydlo R, et al. Somatic variants in epigenetic modifiers can predict failure of response to imatinib but not to second-generation tyrosine kinase inhibitors. Haematologica. 2019;104:2400–9.
Qin YZ, Jiang Q, Jiang H, Lai YY, Zhu HH, Liu YR, et al. Combination of white blood cell count at presentation with molecular response at 3 months better predicts deep molecular responses to imatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Medicine. 2016;95:e2486.
Togasaki E, Takeda J, Yoshida K, Shiozawa Y, Takeuchi M, Oshima M, et al. Frequent somatic mutations in epigenetic regulators in newly diagnosed chronic myeloid leukemia. Blood cancer J. 2017;7:e559.
Zhang XS, Gale RP, Huang XJ, Jiang Q. Is the Sokal or EUTOS long-term survival (ELTS) score a better predictor of responses and outcomes in persons with chronic myeloid leukemia receiving tyrosine-kinase inhibitors? Leukemia. 2022;36:482–91.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99.
Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92.
Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–8.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27:6041–51.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Qin YZ, Jiang Q, Jiang H, Li JL, Li LD, Zhu HH, et al. Which method better evaluates the molecular response in newly diagnosed chronic phase chronic myeloid leukemia patients with imatinib treatment, BCR-ABL(IS) or log reduction from the baseline level? Leuk Res. 2013;37:1035–40.
Guilhot J, Baccarani M, Clark RE, Cervantes F, Guilhot F, Hochhaus A, et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012;119:5963–71.
Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology−with an emphasis on fractional polynomials. Methods Inf Med. 2005;44:561–71.
Sauerbrei W, Meier-Hirmer C, Benner A, Royston PJCS, Analysis D. Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. 2006;50:3464–85.
Kuk D, Varadhan R. Model selection in competing risks regression. Stat Med. 2013;32:3077–88.
Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychological Methods. 2012;17:228–43.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–35.
Hinkley, DV. Bootstrap methods and their application. Bootstrap methods and their application, 1997.
Kulesa A, Krzywinski M, Blainey P, Altman N. Sampling distributions and the bootstrap. Nat Methods. 2015;12:477–8.
Wang S, Wang J, Chung FL. Kernel density estimation, kernel methods, and fast learning in large data sets. IEEE Trans Cybern. 2014;44:1–20.
Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med Res Methodol. 2017;17:53.
Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: From utopia to empirical data. J Clin Epidemiol. 2016;74:167–76.
Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: A guide for investigators. Eur Urol. 2018;74:796–804.
Breccia M, Alimena G. The significance of early, major and stable molecular responses in chronic myeloid leukemia in the imatinib era. Crit Rev Oncol/Hematol. 2011;79:135–43.
Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94:346–57.
Sharf G, Marin C, Bradley JA, Pemberton-Whiteley Z, Bombaci F, Christensen RIO, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34:2102–12.
D’Adda M, Farina M, Schieppati F, Borlenghi E, Bottelli C, Cerqui E, et al. The e13a2 BCR-ABL transcript negatively affects sustained deep molecular response and the achievement of treatment-free remission in patients with chronic myeloid leukemia who receive tyrosine kinase inhibitors. Cancer. 2019;125:1674–82.
Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ, et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94:1362–7.
Qin YZ, Jiang Q, Jiang H, Lai YY, Shi HX, Chen WM, et al. Prevalence and outcomes of uncommon BCR-ABL1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single centre. Br J Haematol. 2018;182:693–700.
Acknowledgements
We thank medical staff and patient participants. QJ acknowledges support from the National Natural Science Foundation of China (No. 81970140). RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. Funded, in part, by the National Nature Science Foundation of China (No. 81970140) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2019-I2M-5-034).
Author information
Authors and Affiliations
Contributions
QJ designed the study. QJ, XSZ, ZYL, and MYZ collected and analyzed the data. QJ, XJH, XSZ, RPG, ZYL, and MYZ prepared the typescript. All authors approved the final typescript, take responsibility for the content, and agree to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
RPG is a consultant to NexImmune Inc. and Ananexa Pharma Ascentage Pharm Group, Antengene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
Ethics approval
The study was approved by the Ethics Committee of People’s Hospital Beijing compliant with principles of the Helsinki Declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Xs., Gale, R.P., Li, Zy. et al. Predictive scoring systems for molecular responses in persons with chronic phase chronic myeloid leukemia receiving initial imatinib therapy. Leukemia 36, 2042–2049 (2022). https://doi.org/10.1038/s41375-022-01616-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01616-y
This article is cited by
-
Allogeneic stem cell transplantation is still a highly curative therapy in adults with philadelphia chromosome–positive acute lymphoblastic leukaemia
Annals of Hematology (2024)
-
External validation of the predictive scoring systems for molecular responses in chronic myeloid leukaemia receiving initial imatinib-therapy
Leukemia (2023)
-
Patients with chronic myeloid leukemia and coronavirus disease 2019 in the Omicron era
Annals of Hematology (2023)