Abstract
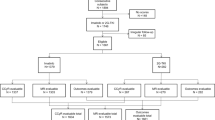
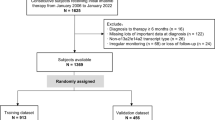
Data from 1,364 consecutive subjects with chronic-phase chronic myeloid leukemia (CML) receiving initial imatinib-therapy were interrogated to identify co-variates predicting therapy failure. Subjects were randomly divided into training (n = 908) and validation datasets (n = 456). In the training dataset, WBC count ≥120 × 10E + 9/L, haemoglobin concentration <115 g/L, blood basophils ≥12% and European Treatment and Outcome Study for CML Long-Term Survival (ELTS) risk score were significantly-associated with failure-free survival (FFS). Each co-variate was assigned 1 point to develop the imatinib-therapy failure (IMTF) model except ELTS high-risk category which was assigned 2 points based on multi-variable regression coefficients. Area under receiver-operator characteristic curve values in the IMTF model for 1-, 3- and 5-year FFS were 0.79–0.84 in the training dataset and 0.78–0.85 in the validation dataset. Calibration plots showed high agreement between predicted and observed outcomes. Decision curve analyses indicated subjects benefited from clinical use of this model. Cumulative incidences of imatinib-therapy failure and probabilities of FFS among the 5 risk cohorts (very low-, low-, intermediate-, high- and very high-risk) using the IMTF model were significantly different (all p values < 0.001). The IMTF model also correlated with probabilities of progression-free survival and survival (all p values < 0.001). These data should help physicians optimize TKI-therapy strategy at diagnosis in persons with chronic phase CML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Berman E. How I Treat chronic phase chronic myelogenous leukemia. Blood. 2021. https://doi.org/10.1182/blood.2021011722 [Online ahead of print].
Nguyen JT, Cole AL, Leech AA, Wood WA, Dusetzina SB. Cost-effectiveness of first-line tyrosine kinase inhibitor therapy initiation strategies for chronic myeloid leukemia. Value Health. 2020;23:1292–9.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Qin YZ, Jiang Q, Jiang H, Li JL, Li LD, Zhu HH, et al. Which method better evaluates the molecular response in newly diagnosed chronic phase chronic myeloid leukemia patients with imatinib treatment, BCR-ABL(IS) or log reduction from the baseline level? Leuk Res. 2013;37:1035–40.
Guilhot J, Baccarani M, Clark RE, Cervantes F, Guilhot F, Hochhaus A, et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012;119:5963–71.
Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–8.
Iba K, Shinozaki T, Maruo K, Noma H. Re-evaluation of the comparative effectiveness of bootstrap-based optimism correction methods in the development of multivariable clinical prediction models. BMC Med Res Methodol. 2021;21:9.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81.
Janssens A, Martens FK. Reflection on modern methods: revisiting the area under the ROC Curve. Int J Epidemiol. 2020;49:1397–403.
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiol. 2010;21:128–38.
Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74:796–804.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–74.
Yu L, Wang H, Gale RP, Qin Y, Lai Y, Shi H, et al. Impact of socio-demographic co-variates on prognosis, tyrosine kinase-inhibitor use and outcomes in persons with newly-diagnosed chronic myeloid leukaemia. J Cancer Res Clin Oncol. 2021.
Erçalışkan A, Seyhan Erdoğan D, Eşkazan AE. Current evidence on the efficacy and safety of generic imatinib in CML and the impact of generics on health care costs. Blood Adv. 2021;5:3344–53.
Efficace F, Stagno F, Iurlo A, Breccia M, Cottone F, Bonifacio M, et al. Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia. 2020;34:488–98.
Kalmanti L, Saussele S, Lauseker M, Müller MC, Dietz CT, Heinrich L, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29:1123–32.
Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404.
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40.
Cortes JE, Khoury HJ, Kantarjian HM, Lipton JH, Kim DW, Schafhausen P, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206–14.
Cortes JE, Kim DW, Kantarjian HM, Brümmendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92.
Cortes JE, Jones D, O’Brien S, Jabbour E, Ravandi F, Koller C, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404.
Cortes JE, Jones D, O’Brien S, Jabbour E, Konopleva M, Ferrajoli A, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–7.
Hehlmann R, Voskanyan A, Lauseker M, Pfirrmann M, Kalmanti L, Rinaldetti S, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020;34:2074–86.
Wang W, Cortes JE, Tang G, Khoury JD, Wang S, Bueso-Ramos CE, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127:2742–50.
Bixby D, Talpaz M. Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia. Leukemia. 2011;25:7–22.
Quintás-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122–31.
Deininger M. Resistance and relapse with imatinib in CML: causes and consequences. J Natl Compr Cancer Netw. 2008;6 Suppl 2:S11–s21.
Shah NP, Rousselot P, Schiffer C, Rea D, Cortes JE, Milone J, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869–74.
Shah NP, Guilhot F, Cortes JE, Schiffer CA, le Coutre P, Brümmendorf TH, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123:2317–24.
Zhang XS, Gale RP, Huang XJ, Jiang Q. Is the Sokal or EUTOS long-term survival (ELTS) score a better predictor of responses and outcomes in persons with chronic myeloid leukemia receiving tyrosine-kinase inhibitors?. Leukemia. 2022;36:482–91.
Liu Z, Shi Y, Yan Z, He Z, Ding B, Tao S, et al. Impact of anemia on the outcomes of chronic phase chronic myeloid leukemia in TKI era. Hematol. 2020;25:181–5.
Baccarani M, Castagnetti F, Gugliotta G, Rosti G, Soverini S, Albeer A, et al. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia. 2019;33:1173–83.
Valent P, Horny HP, Arock M. The underestimated role of basophils in Ph(+) chronic myeloid leukaemia. Eur J Clin Investig. 2018;48:e13000.
Lekovic D, Gotic M, Milic N, Zivojinovic B, Jovanovic J, Colovic N, et al. Predictive parameters for imatinib failure in patients with chronic myeloid leukemia. Hematol. 2017;22:460–6.
Ko PS, Yu YB, Liu YC, Wu YT, Hung MH, Gau JP, et al. Moderate anemia at diagnosis is an independent prognostic marker of the EUTOS, Sokal, and Hasford scores for survival and treatment response in chronic-phase, chronic myeloid leukemia patients with frontline imatinib. Curr Med Res Opin. 2017;33:1737–44.
Qin YZ, Jiang Q, Jiang H, Lai YY, Zhu HH, Liu YR, et al. Combination of white blood cell count at presentation with molecular response at 3 months better predicts deep molecular responses to imatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Medicine. 2016;95:e2486.
Pérez-Jacobo F, Tuna-Aguilar E, Demichelis-Gómez R, Crespo-Solís E, Valencia-Rocha U, Aguayo Á, et al. Prognostic factors, response to treatment, and survival in patients with chronic myeloid leukemia in blast phase: a single-institution survey. Clin Lymphoma Myeloma Leuk. 2015;15:778–84.
Cerny-Reiterer S, Ghanim V, Hoermann G, Aichberger KJ, Herrmann H, Muellauer L, et al. Identification of basophils as a major source of hepatocyte growth factor in chronic myeloid leukemia: a novel mechanism of BCR-ABL1-independent disease progression. Neoplasia. 2012;14:572–84.
Hasford J, Pfirrmann M, Hehlmann R, Baccarani M, Guilhot F, Mahon FX, et al. Prognosis and prognostic factors for patients with chronic myeloid leukemia: nontransplant therapy. Semin Hematol. 2003;40:4–12.
Smulowitz PB, Burke RC, Ostrovsky D, Novack V, Isbell L, Landon BE. Attitudes toward risk among emergency physicians and advanced practice clinicians in Massachusetts. J Am Coll Emerg Phys Open. 2021;2:e12573.
Rodriguez C, Rahman NA, London K, Naples R, Buttar S, Zhang XC, et al. An evaluation of risk attitudes and risk tolerance in emergency medicine residents. Cureus. 2019;11:e4451.
Pines JM, Isserman JA, Szyld D, Dean AJ, McCusker CM, Hollander JE. The effect of physician risk tolerance and the presence of an observation unit on decision making for ED patients with chest pain. Am J Emerg Med. 2010;28:771–9.
Pines JM, Hollander JE, Isserman JA, Chen EH, Dean AJ, Shofer FS, et al. The association between physician risk tolerance and imaging use in abdominal pain. Am J Emerg Med. 2009;27:552–7.
Acknowledgements
Profs. Rüdiger Hehlmann and Markus Pfirrmann kindly reviewed the typescript. We thank medical staff and patient participants. QJ acknowledges support from the National Natural Science Foundation of China (No. 81770161, No. 81970140). RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Funding
Funded, in part, by the National Nature Science Foundation of China (No. 81770161, No. 81970140).
Author information
Authors and Affiliations
Contributions
QJ designed the study. QJ and X-SZ collected and analyzed the data. QJ, X-SZ, RPG, M-JZ and X-JH prepared the typescript. All authors approved the final typescript, take responsibility for the content and agreed to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals; advisor to Antegene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
Ethics approval
The study was approved by the Ethics Committee of People’s Hospital Beijing compliant with principles of the Helsinki Declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, XS., Gale, R.P., Zhang, MJ. et al. A predictive scoring system for therapy-failure in persons with chronic myeloid leukemia receiving initial imatinib therapy. Leukemia 36, 1336–1342 (2022). https://doi.org/10.1038/s41375-022-01527-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01527-y
This article is cited by
-
An MRI-based scoring system for pretreatment risk stratification in locally advanced rectal cancer
British Journal of Cancer (2023)
-
Patients with chronic myeloid leukemia and coronavirus disease 2019 in the Omicron era
Annals of Hematology (2023)
-
Validation of the imatinib-therapy failure model
Leukemia (2023)
-
Real-world experience with ponatinib therapy in chronic phase chronic myeloid leukemia: impact of depth of response on survival and prior exposure to nilotinib on arterial occlusive events
Blood Cancer Journal (2023)