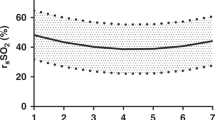
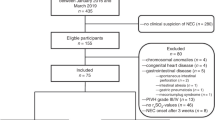
Abstract
Near infrared spectroscopy (NIRS) provides a non-invasive, continuous monitoring of regional tissue oxygenation. NIRS assessment of neonatal splanchnic oxygenation (SrSO2) has gained increasing interest over the last decade, as local hypoxia and ischemia underlie the most feared gut complications in neonates. Current literature provides encouraging evidence in support of SrSO2 reliability in detecting mesenteric hemodynamic changes related to various physiological and pathological conditions in-term and preterm infants. Even so, while splanchnic NIRS monitoring looks promising for investigating gut physiopathology in research settings, further studies are needed to evaluate its feasibility as a routine monitoring tool in neonatal care and to investigate its potential role in clinical decision making. After a brief introduction to NIRS technical principles, this review aims to provide a complete overview of current neonatal applications for splanchnic NIRS monitoring, to discuss its possible limitations and to suggest future directions for research and clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ancel P-Y, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ Gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169:230–8.
Soleymani S, Borzage M, Seri I. Hemodynamic monitoring in neonates: advances and challenges. J Perinatol. 2010;30:S38–S45.
Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy: background and current applications. J Clin Monit Comput. 2012;26:279–87.
Masters B, So P. Handbook of Biomedical Nonlinear Optical Microscopy.. New York: Oxford University Press; 2008.
Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7.
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet. 1986;2:1063–6.
Kurth CD, Steven JL, Montenegro LM, Watzman HM, Gaynor JW, Spray TL, et al. Cerebral oxygen saturation before congenital heart surgery. Ann Thorac Surg. 2001;72:187–92.
Spaeder MC, Klugman D, Skurow-Todd K, Glass P, Jonas RA, Donofrio MT. Perioperative near-infrared spectroscopy monitoring in neonates with congenital heart disease. Pediatr Crit Care Med. 2017;18:213–8.
Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, Savini S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 2013;35:26–31.
Dehaes M, Aggarwal A, Lin P-Y, Rosa Fortuno C, Fenoglio A, Roche-Labarbe N, et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab. 2014;34:87–94.
Sirc J, Dempsey EM, Miletin J. Cerebral tissue oxygenation index, cardiac output and superior vena cava flow in infants with birth weight less than 1250 grams in the first 48 h of life. Early Hum Dev. 2013;89:449–52.
Fujioka T, Takami T, Ishii H, Kondo A, Sunohara D, Kawashima H. Difference in cerebral and peripheral hemodynamics among term and preterm infants during the first three days of life. Neonatology. 2014;106:181–7.
Chaaban H, Stonestreet BS. Intestinal hemodynamics and oxygenation in the perinatal period. Semin Perinatol. 2012;36:260–8.
Balaguru D, Bhalala U, Haghighi M, Norton K. Computed tomography scan measurement of abdominal wall thickness for application of near-infrared spectroscopy probes to monitor regional oxygen saturation index of gastrointestinal and renal circulations in children. Pediatr Crit Care Med. 2011;12:e145–8.
Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33:1433–42.
Matcher SJ, Cope M, Delpy DT. Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys Med Biol. 1994;39:177–96.
Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–R61.
Owen-Reece H, Smith M, Elwell CE, Goldstone JC. Near infrared spectroscopy. Br J Anaesth. 1999;82:418–26.
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–53.
Goff DA, Buckley EM, Durduran T, Wang J, Licht DJ. Noninvasive cerebral perfusion imaging in high-risk neonates. Semin Perinatol. 2010;34:46–56.
Chalia M, Lee CW, Dempsey LA, Edwards AD, Singh H, Michell AW, et al. Hemodynamic response to burst-suppressed and discontinuous electroencephalography activity in infants with hypoxic ischemic encephalopathy. Neurophotonics. 2016;3:31408.
Matcher SJ, Kirkpatrick PJ, Nahid K, Cope M, Delpy DT. Absolute quantification methods in tissue near-infrared spectroscopy. In: Chance B, Alfano RR. International Society for Optics and Photonics, 1995, pp 486–495.
Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–87.
da Costa CS, Greisen G, Austin T. Is near-infrared spectroscopy clinically useful in the preterm infant? Arch Dis Child - Fetal Neonatal Ed. 2015;100:F558–F561.
Naulaers G, Meyns B, Miserez M, Leunens V, Van Huffel S, Casaer P, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology. 2007;92:120–6.
Parks DA, Jacobson ED. Physiology of the splanchnic circulation. Arch Intern Med. 1985;145:1278.
Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:83–7.
Tanis JC, Boelen MR, Schmitz DM, Casarella L, van der Laan ME, Bos AF, et al. Correlation between Doppler flow patterns in growth-restricted fetuses and neonatal circulation. Ultrasound Obstet Gynecol. 2016;48:210–6.
Malcolm G, Ellwood D, Devonald K, Beilby R, Henderson-Smart D. Absent or reversed end diastolic flow velocity in the umbilical artery and necrotising enterocolitis. Arch Dis Child. 1991;66:805–7.
Kamoji VM, Dorling JS, Manktelow B, Draper ES, Field DJ. Antenatal umbilical Doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatr Int J Paediatr. 2008;97:327–31.
Westby Eger S, Kessler J, Kiserud T, Markestad T, Sommerfelt K. Foetal Doppler abnormality is associated with increased risk of sepsis and necrotising enterocolitis in preterm infants. Acta Paediatr. 2015;104:368–76.
Young CM, Kingma SDK, Neu J. Ischemia-reperfusion and neonatal intestinal injury. J Pediatr. 2011;158:e25–8.
Fujii AM, Brown E, Mirochnick M, O’Brien S, Kaufman G. Neonatal necrotizing enterocolitis with intestinal perforation in extremely premature infants receiving early indomethacin treatment for patent ductus arteriosus. J Perinatol. 2002;22:535–40.
Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabañas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: analysis of systemic effects and neonatal clinical outcomes. Pediatrics. 2006;117:e1213–e1222.
Milner ME, de la Monte SM, Moore GW, Hutchins GM. Risk factors for developing and dying from necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1986;5:359–64.
Rand T, Weninger M, Kohlhauser C, Bischof S, Heinz-Peer G, Trattnig S, et al. Effects of umbilical arterial catheterization on mesenteric hemodynamics. Pediatr Radiol. 1996;26:435–8.
Lane AJ, Coombs RC, Evans DH, Levin RJ. Effect of feed interval and feed type on splanchnic haemodynamics. Arch Dis Child Fetal Neonatal Ed. 1998;79:F49–53.
Bozzetti V, Paterlini G, De Lorenzo P, Gazzolo D, Valsecchi MG, Tagliabue PE. Impact of continuous vs bolus feeding on splanchnic perfusion in very low birth weight infants: a randomized trial. J Pediatr. 2016;176:86–92.e2.
Gladman G, Sims DG, Chiswick ML. Gastrointestinal blood flow velocity after the first feed. Arch Dis Child. 1991;66:17–20.
Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001;85:F42–5.
Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GCS, Kempley ST. Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. 2006;118:1999–2003.
Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Doppler assessment of human neonatal gut blood flow velocities: postnatal adaptation and response to feeds. J Pediatr Gastroenterol Nutr. 1992;15:6–12.
Kempley ST, Gamsu HR. Superior mesenteric artery blood flow velocity in necrotising enterocolitis. Arch Dis Child. 1992;67:793–6.
Bailey SM, Mally PV. Review of splanchnic oximetry in clinical medicine. J Biomed Opt. 2016;21:91306.
McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. 2011;31:51–7.
Schat TE, van der Laan ME, Schurink M, Hulscher JBF, Hulzebos CV, Bos AF, et al. Abdominal near-infrared spectroscopy in preterm infants: A comparison of splanchnic oxygen saturation measurements at two abdominal locations. Early Hum Dev. 2014;90:371–5.
Gay AN, Lazar DA, Stoll B, Naik-Mathuria B, Mushin OP, Rodriguez MA, et al. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg. 2011;46:1034–40.
Gillam-Krakauer M, Cochran CM, Slaughter JC, Polavarapu S, McElroy SJ, Hernanz-Schulman M, et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J Perinatol. 2013;33:609–12.
Lucas A, Bloom SR, Aynsley-Green A. Postnatal surges in plasma gut hormones in term and preterm infants. Biol Neonate. 1982;41:63–7.
Grisoni ER, Kalhan SC. Plasma vasoactive intestinal polypeptide in the newborn infant. J Pediatr Gastroenterol Nutr. 1990;10:185–8.
Dave V, Brion LP, Campbell DE, Scheiner M, Raab C, Nafday SM. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol. 2009;29:213–218.
Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998;102:337–41.
Aynsley-Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatr Scand. 1982;71:379–383.
Dani C, Pratesi S, Barp J, Bertini G, Gozzini E, Mele L, et al. Near-infrared spectroscopy measurements of splanchnic tissue oxygenation during continuous versus intermittent feeding method in preterm infants. J Pediatr Gastroenterol Nutr. 2013;56:652–6.
Corvaglia L. MSBBRPAAFG. Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr Res. 2014;76:81–5.
Bora R, Mukhopadhyay K, Saxena AK, Jain V, Narang A. Prediction of feed intolerance and necrotizing enterocolitis in neonates with absent end diastolic flow in umbilical artery and the correlation of feed intolerance with postnatal superior mesenteric artery flow. J Matern Fetal Neonatal Med. 2009;22:1092–6.
Corvaglia L, Martini S, Battistini B, Rucci P, Faldella G, Aceti A. Splanchnic oxygenation at first enteral feeding in preterm infants. J Pediatr Gastroenterol Nutr. 2017;64:550–4.
Martini S, Aceti A, Beghetti I, Faldella G, Corvaglia L. Feed-related splanchnic oxygenation in preterm infants with abnormal antenatal doppler developing gut complications. J Pediatr Gastroenterol Nutr 2017; Nov 3 [Epub ahead of print].
Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Abnormal gut blood flow velocities in neonates at risk of necrotising enterocolitis. J Pediatr Gastroenterol Nutr. 1992;15:13–9.
Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med. 2011;24:574–82.
Zabaneh RN, Cleary JP, Lieber CA. Mesentric oxygen saturations in premature twins with and without necrotizing enterocolitis. Pediatr Crit Care Med. 2011;12:e404–6.
Stapleton GE, Eble BK, Dickerson HA, Andropoulos DB, Chang AC. Mesenteric oxygen desaturation in an infant with congenital heart disease and necrotizing enterocolitis. Tex Hear Inst J. 2007;34:442–4.
Patel AK, Lazar DA, Burrin DG, Smith EO, Magliaro TJ, Stark AR, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. 2014;15:735–41.
Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001;27:1401–7.
Schat TE, Heida FH, Schurink M, van der Laan ME, Hulzebos CV, Bos AF, et al. The relation between splanchnic ischaemia and intestinal damage in necrotising enterocolitis. Arch Dis Child - Fetal Neonatal Ed. 2016;101:F533–F539.
van der Laan ME, Schat TE, Olthuis AJ, Boezen HM, Bos AF, Kooi EMW. The association between multisite near-infrared spectroscopy and routine hemodynamic measurements in relation to short-term outcome in preterms with clinical sepsis. Neonatology. 2015;108:297–304.
van der Laan ME, Roofthooft MTR, Fries MWA, Schat TE, Bos AF, Berger RMF, et al. Multisite tissue oxygenation monitoring indicates organ-specific flow distribution and oxygen delivery related to low cardiac output in preterm infants with clinical sepsis. Pediatr Crit Care Med. 2016;17:764–71.
Bailey S, Hendricks-Muñoz K, Wells J, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. 2010;27:445–53.
Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220–6.
Sandal G, Oguz SS, Erdeve O, Akar M, Uras N, Dilmen U. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion. 2014;54:1100–5.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal Perinat Med. 2014;7:89–100.
White L, Said M, Rais-Bahrami K. Monitoring mesenteric tissue oxygenation with near-infrared spectroscopy during packed red blood cell transfusion in preterm infants. J Neonatal Perinat Med. 2015;8:157–63.
Banerjee J, Leung TS, Aladangady N. Blood transfusion in preterm infants improves intestinal tissue oxygenation without alteration in blood flow. Vox Sang. 2016;111:399–408.
Banerjee J, Leung TS, Aladangady N. Effect of blood transfusion on intestinal blood flow and oxygenation in extremely preterm infants during first week of life. Transfusion. 2016;56:808–15.
White L, Said M, Rais-Bahrami K. Monitoring mesenteric tissue oxygenation with near-infrared spectroscopy during packed red blood cell transfusion in preterm infants. J Neonatal Perinat Med. 2015;8:157–63.
Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol. 2009;26:99–105.
Marin T, Josephson CD, Kosmetatos N, Higgins M, Moore JE. Feeding preterm infants during red blood cell transfusion is associated with a decline in postprandial mesenteric oxygenation. J Pediatr. 2014;165:464–71.e1.
Bailey SM, Hendricks-Muñoz KD, Mally PV. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood Transfus. 2015;13:429–34.
Marin T, Moore J, Kosmetatos N, Roback JD, Weiss P, Higgins M, et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birth-weight infants: a near-infrared spectroscopy investigation. Transfusion. 2013;53:2650–8.
Stothers L, Shadgan B, Macnab A. Urological applications of near infrared spectroscopy. Can J Urol. 2008;15:4399–409.
Thompson A, Benni P, Seyhan S, Ehrenkranz R. Meconium and transitional stools may cause interference with near-infrared spectroscopy measurements of intestinal oxygen saturation in preterm infants. In: Advances in experimental medicine and biology. 2013, pp 287–292.
Said MM, Niforatos N, Rais-Bahrami K. Validation of near infrared spectroscopy to measure abdominal somatic tissue oxygen saturation in neonates. J Neonatal Perinat Med. 2013;6:23–30.
Akotia DH, Durham JT, Arnell KM, Petruzzelli DL, Katheria AC. Relationship between near-infrared spectroscopy and transabdominal ultrasonography: noninvasive monitoring of intestinal function in neonates. Med Sci Monit. 2016;22:61–8.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J Neonatal Perinat Med. 2014;7:199–206.
Bozzetti V, Paterlini G, Bel Fv, Visser GH, Tosetti L, Gazzolo D, Tagliabue PE. Cerebral and somatic NIRS-determined oxygenation in IUGR preterm infants during transition. J Matern Fetal Neonatal Med. 2016;29:443–6
Richter AE, Schat TE, Van Braeckel KNJA, Scherjon SA, Bos AF, Kooi EMW. The effect of maternal antihypertensive drugs on the cerebral, renal and splanchnic tissue oxygen extraction of preterm neonates. Neonatology. 2016;110:163–71.
Nachar RA, Booth EA, Friedlich P, Borzage M, Soleymani S, Wider MD, et al. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr Res. 2011;70:473–9.
Petrova A, Bhatt M, Mehta R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J Perinatol. 2011;31:460–4.
Cerbo RM, Cabano R, Di Comite A, Longo S, Maragliano R, Stronati M. Cerebral and somatic rSO2 in sick preterm infants. J Matern Fetal Neonatal Med. 2012;25:97–100.
Ledo A, Aguar M, Núñez-Ramiro A, Saénz P, Vento M. Abdominal near-infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology. 2017;112:238–45.
Wassenaar EB, Van, den Brand JGH. Reliability of near-infrared spectroscopy in people with dark skin pigmentation. J Clin Monit Comput. 2005;19:195–9.
Thompson DK, Chen J, Beare R, Adamson CL, Ellis R, Ahmadzai ZM, et al. Structural connectivity relates to perinatal factors and functional impairment at 7years in children born very preterm. Neuroimage. 2016;134:328–37.
Demirel G, Oguz SS, Celik IH, Erdeve O, Dilmen U. Cerebral and mesenteric tissue oxygenation by positional changes in very low birth weight premature infants. Early Hum Dev. 2012;88:409–11.
Bhatt M, Petrova A, Mehta R. Does treatment of patent ductus arteriosus with cyclooxygenase inhibitors affect neonatal regional tissue oxygenation? Pediatr Cardiol. 2012;33:1307–14.
Guzoglu N, Sari FN, Ozdemir R, Oguz SS, Uras N, Altug N, et al. Renal and mesenteric tissue oxygenation in preterm infants treated with oral ibuprofen. J Matern Neonatal Med. 2014;27:197–203.
Montaldo P, De Leonibus C, Giordano L, De Vivo M, Giliberti P. Cerebral, renal and mesenteric regional oxygen saturation of term infants during transition. J Pediatr Surg. 2015;50:1273–7.
Bailey SM, Hendricks-Munoz KD, Mally P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am J Perinatol. 2014;31:339–44.
Azhibekov T, Noori S, Soleymani S, Seri I. Transitional cardiovascular physiology and comprehensive hemodynamic monitoring in the neonate: Relevance to research and clinical care. Semin Fetal Neonatal Med. 2014;19:45–53.
Pocivalnik M, Pichler G, Zotter H, Tax N, Müller W, Urlesberger B. Regional tissue oxygen saturation: comparability and reproducibility of different devices. J Biomed Opt. 2011;16:057004.
Schneider A, Minnich B, Hofstätter E, Weisser C, Hattinger-Jürgenssen E, Wald M. Comparison of four near-infrared spectroscopy devices shows that they are only suitable for monitoring cerebral oxygenation trends in preterm infants. Acta Paediatr. 2014;103:934–8.
Hessel TW, Hyttel-Sorensen S, Greisen G. Cerebral oxygenation after birth - a comparison of INVOS(®) and FORE-SIGHTTM near-infrared spectroscopy oximeters. Acta Paediatr. 2014;103:488–93.
Dix LML, van Bel F, Baerts W, Lemmers PMA. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res. 2013;74:557–63.
Acknowledgements
Funding:
No financial assistance was received in support of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Martini, S., Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J Perinatol 38, 431–443 (2018). https://doi.org/10.1038/s41372-018-0075-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0075-1
This article is cited by
-
The effect of drip versus intermittent feeding on splanchnic oxygenation in preterm infants with intrauterine growth restriction: a prospective randomized trial
European Journal of Pediatrics (2023)
-
Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?
Pediatric Research (2023)
-
Vital signs as physiomarkers of neonatal sepsis
Pediatric Research (2022)
-
Renal oxygenation measured by near-infrared spectroscopy in preterm neonates in the first week
Pediatric Research (2022)
-
Splanchnic oxygen saturation during reoxygenation with 21% or 100% O2 in newborn piglets
Pediatric Research (2022)