Abstract
Background/objectives
Weight gain increases risk of cardiovascular disease, but has not been examined extensively in relationship to venous thromboembolism (VTE). The association between weight change over 9 years and subsequent VTE among participants in the Atherosclerosis Risk in Communities (ARIC) study was examined, with a hypothesis that excess weight gain is a risk factor for VTE, relative to no weight change.
Subjects/methods
Quintiles of 9-year weight change were calculated (visit 4 1996–1998 weight minus visit 1 1987–1989 weight in kg: Quintile 1: ≥−1.81 kg; Quintile 2: <−1.81 to ≤1.36 kg; Quintile 3: >1.36 to ≤4.08 kg; Quintile 4: >4.08 to ≤7.71 kg; Quintile 5: >7.71 kg). Incident VTEs from visit 4 (1996–1998) through 2015 were identified and adjudicated using medical records. Hazard ratios (HRs) were calculated using Cox models.
Results
529 incident VTEs were identified during an average of 19 years of follow up. Compared to Quintile 2, participants in Quintile 5 of weight change had 1.46 times the rate of incident VTE (HR = 1.46 (95% CI 1.09, 1.95), adjusted for age, race, sex, income, physical activity, smoking, and prevalent CVD). The HR for Quintile 5 was modestly attenuated to 1.38 (95% CI 1.03, 1.84) when visit 1 BMI was included in the model. When examined separately, results were significant for unprovoked VTE, but not for provoked VTE. Among those obese at visit 1, both weight gain (HR 1.86 95% CI 1.27, 2.71) and weight loss (HR 2.11 95% CI 1.39, 3.19) were associated with incident VTE, compared with normal-weight participants with no weight change.
Conclusions
Weight gain later life was associated with increased risk for unprovoked VTE. Among those with obesity, both weight gain and weight loss were associated with increased risk for VTE.
Similar content being viewed by others
Introduction
Deep venous thrombosis and pulmonary embolism, collectively, venous thromboembolism (VTE), is a life-threatening disease and a serious public health issue [1,2,3]. It affects almost more than 1 million Americans annually [4] and results in ~100,000 deaths [1], making it the third leading cause of cardiovascular mortality behind heart attack and stroke [4, 5].
Obesity is a well-established independent risk factor for VTE [4, 6,7,8,9]. People with obesity (body mass index BMI >= 30 kg/m2) have 2–3 times higher risk of VTE compared with people with normal weight [3, 6, 8]. This association is observed consistently across various measures of obesity, including BMI, waist circumference, waist-hip-ratio, and calf circumference [6, 7, 10, 11], and persists after adjustment for other VTE risk factors [6, 7, 9, 10].
Adult weight gain has adverse effects on both arterial vascular and VTE risk factors [12,13,14,15,16,17]. A possible hypothesis is that adult weight gain increases risk for incident VTE, as it does for incident arterial vascular disease. Although many studies show an association between a single weight measure with VTE, we are aware of only one study that examined weight change and VTE risk [18]. In the Tromsø Study, weight gain of 7.5 kg or more over 6 years was associated with a 1.9-fold higher incidence of subsequent VTE compared to weight gain <7.5 kg [18]. The highest risk of VTE was observed among those with BMI >= 30 at baseline who gained any weight (6.6-fold greater VTE risk compared to normal-weight people with no weight gain).
The present study is the first US study to examine the prospective association between weight change over 19 years and incident VTE, using data from participants in the Atherosclerosis Risk in Communities (ARIC) study [19]. We hypothesized that greater weight gain would be associated with increased risk of VTE, and that the highest risk of incident VTE would be observed among participants who were obese at baseline and gained appreciable weight.
Methods
Study design and participants
The ARIC cohort includes N = 15,792 study participants aged 45–64 years from four US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland) [19]. Following clinic visit 1 in 1987–1989, participants were contacted annually or semi-annually by telephone, and periodic in-person clinical follow up visits were conducted, including visit 2 (1990–1992), visit 3 (1993–1995), and visit 4 (1996–1998). The present analysis focused on cohort members who attended visit 4 (N = 11,656). Follow-up time began at visit 4 and continued to either incident VTE, loss to follow-up, death, or administrative censoring at December 31, 2015. IRB approval for ARIC was received by the Human Subjects Protection Board at each of the participating universities.
Measures
Body weight change
Weight and height were measured according to standardized protocols at every clinic visit [19]. BMI was calculated as weight in a scrub suit in kg/height in m2. BMI categories were defined as normal weight, overweight, and obese by BMI < 25 kg/m2, 25 kg/m2 to <30 kg/m2 and >=30 kg/m2. Quintiles of 19-year weight change were calculated (visit 4 1996–1998 weight minus visit 1 1987–1989 weight in kg): Quintile 1: ≥−1.81 kg; Quintile 2 (referent): <−1.81 to ≤1.36 kg; Quintile 3: >1.36 to ≤4.08 kg; Quintile 4: >4.08 to ≤7.71 kg; Quintile 5: >7.71 kg. Quintile 2 was selected as the referent because it includes the “no weight change” group, and small loss and small gain. Since small weight gain is typical in population-based cohorts, this quintile was viewed as the most appropriate to serve as a reference group [14,15,16,17, 21, 22]. We also categorized weight change using broader categories for some analyses (described below) where sparse data were a concern: weight loss (>−2.7 kg); no change (−2.7 kg to +2.7 kg); weight gain (>+2.7 kg).
Covariates
Age, sex, race, prevalent cardiovascular disease, and smoking status (never, former or current smoker) were self-reported. Race-center (Forsyth County whites, Forsyth County blacks, Jackson blacks, Minneapolis whites, and Washington County blacks) was included as a covariate due to differences in sample racial composition at ARIC sites. Physical activity was measured at visit 1 and visit 3 using the self-report Baecke questionnaire [20] Participants self-reported the types and frequency of sports currently engaged in, and a sports score was computed using MET codes for specific types of activities.
Venous thromboembolism ascertainment
Hospitalizations were identified by participant report during follow-up calls and through systematic searches of local hospital discharges for cohort members. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or ICD-10-CM discharge codes of hospitalizations were recorded and used to identify possible cases of VTE from visit 4 through 2015.
To classify VTE occurrence, information from hospital records for each potential VTE was reviewed independently by two physicians (MC, ARF), as previously described [3]. Differences in classification were resolved by discussion. Clinical diagnoses without objective tests were not validated as cases. Definite deep vein thrombosis was defined as a positive duplex ultrasound or venogram or, in rare cases, by computed tomography or impedance plethysmography. Pulmonary embolism was classified using results of computed tomography, ventilation/perfusion scans, or, rarely, angiography or autopsy.
VTEs also were classified as unprovoked or provoked (occurring within 90 days of major trauma, surgery, or marked immobility, or associated with active cancer or chemotherapy). Venous thromboses not in the leg or vena cava were not considered as VTE for this analysis.
Statistical analysis
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% confidence intervals for incident VTE for quintiles of weight change, using quintile 2 as the referent category. Model 1 adjusted for age, race-center, and sex; model 2 adjusted for these variables plus income at visit 4, smoking status at visit 1 and 4, physical activity at visit 1 and 3, and prevalent cardiovascular disease at visit 1; and model 3 adjusted for model 2 variables plus visit 1 BMI.
Additional analyses examined the associations between weight change category (loss, no change, gain) and incident VTE stratified by visit 1 body mass index (normal weight; overweight; obese). Categories of weight change (defined above) were used in this analysis for ease of presentation and interpretation. Interactions between baseline weight and weight change category over time were tested using cross-product terms.
Sensitivity analyses were conducted to examine results (i) for provoked and unprovoked VTE separately and (ii) additionally adjusting for post visit 4 (time-varying) use of anticoagulants (results were not affected and are not shown).
Results
Description of the cohort at Visit 4
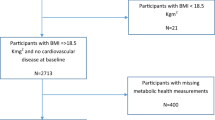
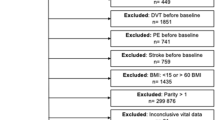
Among ARIC participants who attended visit 4 in 1996–1998 (N = 11,656; 73.8% of initial ARIC cohort), the following exclusions were made: prevalent VTE at visit 4 (N = 339); anticoagulant use at visit 4 (N = 236); prevalent cancer self-reported at visit 1 or visit 4 (N = 1404); missing visit 1 or visit 4 weight (N = 42); or not White or Black at certain ARIC Centers (N = 69; see above “Covariates”). The final analytic cohort (N = 9710) had a higher proportion of Blacks (24% versus 16%) and was slightly younger (62 years versus 64 years), compared to those excluded from analysis.
At visit 4, included participants averaged 62 years and had BMI 29.6 kg/m2; 56% were female, 24% were Black, 17% had diabetes, and 15% were current smokers. Table 1 shows participant characteristics by quintiles of weight change from visit 1 to visit 4. Participants who had gained greater weight were more likely to be female, younger, and former smokers at visit 4, compared to those in the lower quintiles of weight gain.
Weight change and incident VTE
Over an average of 19.4 years (sd = 4.7) of follow-up, 526 incident VTEs were identified. Table 2 shows weight change quintiles and hazard ratios for VTE. In an adjusted model (model 2), relative to Quintile 2, the Quintile 5 (weight gain of >+7.7 kg) HR was 1.46 (95% CI 1.09, 1.95) for incident VTE. In analysis of provoked VTE, compared with Quintile 2 of weight change, neither greater weight gain nor loss tended to be associated with VTE (Table 2). However, a large weight gain (>+7.71 kg, Quintile 5) was associated with a greater incidence of unprovoked VTE compared to Quintile 2 of weight change (adjusted model 2: HR 2.03, (95% CI 1.29, 3.19)). Although slightly weaker, weight loss (Quintile 1) was also associated with greater incidence of unprovoked VTE compared with Quintile 2 (adjusted model 2: HR 1.61 (95% CI 1.01, 2.56)).
Among the normal weight at visit 1, those who gained weight were at risk of VTE comparable to those who did not gain weight (Table 3). Among those obese at visit 1, weight gain >+2.7 kg was associated with an adjusted (model 2) HR of 1.86 (95% CI 1.27, 2,71) compared with those of normal weight at visit 1 who did not gain weight. The crude incidence rate among those obese at visit 1 who gained weight was 5.14/1000 PY, compared with 2.47/1000 PY among those of normal weight at visit 1 who did not gain weight. Among those obese at visit 1, weight loss also was significantly associated with an adjusted (model 2) HR for VTE of 2.11 (95% CI 1.39, 3.19) compared with those normal weight at visit 1 with no weight change.
Discussion
This research demonstrated a modest association between a large weight gain over 9 years and greater future unprovoked VTE in a large cohort of black and white adults with average age 62 years over an average follow up of 19 years. Specifically, those who gained more than 7.7 kg, compared to those who lost up to −1.8 kg or gained up to 1.4 kg, had 1.46 times the rate of VTE after adjusting for risk factors, while the rate was 2.03 times higher for unprovoked VTE. It was hypothesized that the risk of VTE would be more pronounced among participants with obesity at visit 1 who gained significant weight during the follow up. Relative to normal-weight participants who did not gain weight, obese participants who gained +2.7 kg or more had 1.86 times the rate of VTE risk during follow up, after adjustment for income, smoking, and physical activity. Obese participants who lost weight were also at 2.11 times higher risk for VTE compared with normal-weight participants who did not gain weight.
A lack of association of weight gain with provoked VTE suggests that causes of provoked VTE, such as underlying disease or immobility, are more relevant pathophysiologically than weight gain. Alternatively, it may be that these conditions over the 19-year weight change period caused weight change and thus confounded the analysis of provoked VTE.
In the present study, excess weight gain seemed to be a significant risk factor for VTE, regardless of initial body weight, although associations for weight gain were attenuated by adjustment for starting BMI. These findings are inconsistent with the Tromsø results, where risk for VTE with weight gain was magnified among those who were already obese at the start of the observation period [18]. Differences in VTE incidence rates, follow up length, and weight change category definitions may explain some of the differences in the results between the two studies. For example, the Tromso study (average baseline age 59 years) had a 6 year follow up period and the present ARIC analyses (average visit 4 age 62 years) had a 19 year follow up period; crude VTE incidence rate was 1.8/1000 PY in Tromsø [18] and 3.5/1000 PY in the present ARIC analyses, and Tromsø defined no weight change as a gain of 0 through +7.4, compared to the present study’s definition of a loss of −2.7 through a gain of +2.7 kg. Among those obese at visit 1, Tromsø observed a HR of 6.6 (95% CI 3.61, 12.22) of VTE in adjusted models with the referent of normal weight with no weight change. In the present study, the HR for those obese at visit 1 was 1.86 (95% CI 1.27, 2.71) in adjusted models with the same referent group of normal weight with no weight change. Additional studies are needed to clarify whether, independent of initial BMI, excess weight gain is an independent risk factor for both provoked and unprovoked VTE.
The present study had several strengths, such as the inclusion of black and white participants across four diverse US geographic locations, a lengthy follow-up duration of 19 years, and a large number of incident and adjudicated VTEs. A limitation is a lack of data on intentionality of weight change. Previous research on the association between weight change and health outcomes has largely shown that unintentional weight loss, but intentional weight loss less so, is associated with greater subsequent disease onset and mortality [15, 21, 22]. Another limitation is that other factors that impact weight gain, such as physical activity, are measured with error which could result in residual confounding.
These data provide evidence that a large weight gain later in life contributes to increase risk for unprovoked VTE. Among those with existing obesity, large gain or loss is associated with increased risk of unprovoked VTE.
References
US Department of Health and Human Services. The Surgeon General’s Call to Action to prevent deep vein thrombosis and pulmonary embolism. Rockville, MD: Office of the Surgeon General (US), National Heart, Lung and Blood Institute; 2008.
Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14.
Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25.
Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, et al. Heart disease and stroke statistics - 2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528.
Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–46.
Braekkan SK, Siegerink B, Lijfering WM, Hansen J-B, Cannegieter SC, Rosendaal FR. Role of obesity in the etiology of deep vein thrombosis and pulmonary embolism: current epidemiological insights. Semin Thromb Hemost. 2013;39:533–40.
Horvei LD, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen J-B. Obesity measures and risk of venous thromboembolism and myocardial infarction. Eur J Epidemiol. 2014;29:821–30.
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta analysis. Circulation. 2008;117:93–102.
Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–903.
Cushman M, M’Meara ES, Heckbert SR, Zakai NA, Rosamond W, Folsom AR. Body size measures, hemostatic and inflammatory markers and risk of venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Thromb Res. 2016;144:127–32.
Olson NC, Cushman M, Lutsey PL, McClure LA, Judd S, Tracy RP, et al. Inflammation markers and incident venous thromboembolism: the REasons for geographic and racial differences in stroke (REGARDS) cohort. J Thromb Haemost. 2014;12:1993–2001.
Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6.
Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–8.
Lissner L, Odell PM, D’Agostino RB, Stokes J, Kreger BE, Belanger AJ, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–44.
French SA, Jeffery RW, Folsom AR, Williamson DF, Byers T. Relation of weight variability and intentionality of weight loss to disease history and health-related variables in a population-based sample of women aged 55–69 years. Am J Epidemiol. 1995;142:1306–14.
Folsom AR, French SA, Zheng W, Baxter JE, Jeffery RW. Weight variability and mortality: the Iowa Women’s Health Study. Intl J Obesity Relat Metab Disord. 1996;20:704–9.
Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the’normal’ weight range. JAMA. 1995;273:461–5.
Horvel LD, Braekkan SK, Hansen J-B. Weight change and risk of venous thromboembolism: the Tromso Study. PLoS ONE 206;11:e0168878.
The ARIC Investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702.
Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42.
Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS ONE. 2015;10:e0121993.
French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women’s Health Study. Am J Epidemiol. 1999;149:504–14.
Acknowledgements
The National Heart, Lung, and Blood Institute provided support for the ARIC study via contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I and for this venous thromboembolism research via R01HL059367. We thank the ARIC participants and staff for their important contributions to ARIC research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
French, S.A., Lutsey, P.L., Rosamond, W. et al. Weight change over 9 years and subsequent risk of venous thromboembolism in the ARIC cohort. Int J Obes 44, 2465–2471 (2020). https://doi.org/10.1038/s41366-020-00674-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00674-5
This article is cited by
-
Epidemiology and prevention of venous thromboembolism
Nature Reviews Cardiology (2023)