Abstract
Mucopolysaccharidosis IVA (MPS IVA) is a degenerative systemic skeletal dysplasia, in which children exhibit marked short stature and become physically handicapped. This study evaluated the growth patterns of patients treated with enzyme replacement therapy (ERT), compared with those of untreated patients. Cross-sectional and longitudinal data of heights and weights were collected from 128 MPS IVA patients and compared with the growth charts of MPS IVA. Twelve patients (six males, six females) starting ERT before 5 years old were treated for at least 2 years. Six out of 12 patients (50%) with ERT over 2 years stopped growing between 94 and 98 cm (mean height of 95.1 ± 2.2 cm) from 5.0 years to 9.0 years of age (mean age of 6.2 ± 1.6 years). The other patients, except one attenuated case, exhibited a marked slow growth velocity from 3.6 years to 7.7 years. Treated and untreated patients with severe phenotype reached their final heights by ~10 years of age. Patients treated with ERT exhibited a reduced pubertal growth spurt analogous to their untreated counterparts, which contributes to the marked short stature associated with MPS IVA. Compared with the growth charts for untreated patients, patients treated with ERT did not show any significant increase in growth in any age group. Overall, ERT-treated patients do not experience growth improvement and continue to exhibit poor growth despite early ERT intervention before 5 years of age. These findings indicate that current intravenous ERT is ineffective at correcting abnormal growth in MPS IVA.
Similar content being viewed by others
Introduction
Mucopolysaccharidosis IVA (Morquio A syndrome; MPS IVA) is a rare autosomal recessive disorder, caused by the deficiency of the lysosomal enzyme, N-acetylgalactosamine-6-sulfate sulfatase (GALNS) [1,2,3,4,5,6,7,8]. This enzyme is required for the catabolism of the glycosaminoglycans (GAGs), chondroitin-6-sulfate (C6S) and keratan sulfate (KS). The absence of GALNS blocks the degradation of C6S and KS, leading to the accumulation of the partially degraded GAGs, mainly in bone, cartilage, ligaments, and meniscus. Several studies have documented the incidence of MPS IVA, with the highest being 1 in 76,000 live births in Northern Ireland [1, 5, 9], 1 in 200,000 live births in British Columbia and Australia [5, 10, 11], 1 in 450,000 live births in The Netherlands and Portugal, and 500,000 live births in Japan [1, 5, 12,13,14,15].
MPS IVA patients develop unique skeletal manifestations due to an imbalance of growth including a prominent forehead, tracheal obstruction, short neck, pectus carinatum, short trunk dwarfism, kyphosis, scoliosis, genu valgum, coxa valga, and laxity of joints [1,2,3,4,5,6, 16,17,18,19]. Most affected individuals appear normal at birth but develop initial clinical manifestations within the first couple of years of life [1, 4, 6, 20]. Individuals with MPS IVA exhibit a range in phenotypes, from attenuated to severe. Systemic bone involvement increases with the severity of the disease phenotype [1, 2, 4]. In severe phenotypes, instability of the C1–C2 joint, spinal cord compression, and dysplasia of hip and knee joints require multiple orthopedic surgeries [2, 5, 19, 21].
Therapies for MPS IVA have been developed including hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) [22, 23]. HSCT for MPS IVA was approved in Japan in 1995, based on the successful transplantation and its effect [24, 25]. Conventional ERT for MPS IVA was approved in Europe and in the United States in 2014, with the results of a 24-week clinical trial, which showed an improvement of 22.5 m from the baseline in the 6-min walk test (6MWT) and a reduction of urinary KS by 40.7% [26]. MPS IVA patients exhibit difficulties with ambulation primarily due to skeletal dysplasia. The 6MWT is influenced by the musculoskeletal, respiratory, and cardiovascular systems. The test cannot be performed in children <5–6 years of age due to their inability to fully understand how to perform the test. Performance in the test is influenced by the site of the test, training, willingness to cooperate, and stage of development [23].
Most GALNS enzymes used in intravenous ERT are delivered to visceral organs; liver and spleen [22, 23, 27]. It has been shown that the infused enzyme can hardly reach the avascular growth plate [28]. Until now, ERT for MPS IVA has not shown improvement in patients with hypermobile joints, skeletal dysplasia, or bone pathology [6, 22, 29, 30], as observed in MPS IVA mice treated with ERT [28, 31]. Thus, there is a lack of evidence that the current ERT provides a significant impact on bone pathology in MPS IVA, even after long-term treatment [22, 23, 28].
There have also been several additional limitations with intravenous ERT for treatment of MPS IVA, including (i) rapid clearance from circulation and a short half-life (40 min for humans, and 2.9 min for mice) [23, 28, 32], (ii) high cost (~$500,000 per 25 kg patient) [22, 23], (iii) limited effect on corneal, heart valvular, and previously mentioned skeletal issues [23, 33, 34], and (iv) immunological response [35, 36]. Thus, developing therapies for resolving bone lesions remains an unmet challenge [23, 28, 37,38,39,40].
As MPS IVA remains a skeletal disorder primarily, short stature affects the activity of daily living (ADL) of patients [1, 4, 41], hearing loss [42], and tracheal obstruction [19, 30]. Growth impairment in children with classical MPS IVA begins in early childhood; however, some patients with the attenuated phenotype may continue to grow into their teens [1, 4, 5]. The determination of clinical severity in MPS IVA is currently based on the growth of patients [1, 4]. The growth and final height of MPS IVA patients differ markedly from healthy children, and therefore, standard growth charts should not be used for children with MPS IVA [4]. It is essential to assess the growth of MPS IVA patients to monitor disease progression, clinical severity, and response to treatment by using the established growth charts for MPS IVA patients [1].
In this article, we describe the growth of MPS IVA patients treated with ERT, compared to that in untreated MPS IVA patients to evaluate the effectiveness of ERT.
Materials and methods
Study subjects
This study was based on the data collected from 128 MPS IVA patients (68 females and 60 males) at Nemours/Alfred I. duPont Hospital for Children and Gifu University. Seventy-four patients were of Caucasian origin, 48 from Asian origin, and 6 from African American origin. Patients who received ERT were treated with weekly infusions of elosulfase alfa (2.0 mg/kg). Sixty-seven MPS IVA patients were treated with ERT, and 61 patients were untreated. Height measurements were performed in a standing position, if possible, or the patient lied on a flat surface with knees flattened to extend the legs fully. Height measurement values in patients over 18 years of age were considered unchanged. Thus, the data from MPS IVA patients over the age of 18 years were grouped as data at 18 years. The reference curves obtained from height and weight measurements in MPS IVA patients were compared with the established growth charts for MPS IVA patients [1]. We compared the reference curves of MPS IVA patients to those of healthy children provided by the Centers for Disease Control (CDC) as well. The study was approved by the local IRB committee (IRB #: 750932, IRB #: 277662). Informed consent was obtained from each patient or the caregiver.
Statistical analysis
The data used in the construction of the growth charts included age (months and years), height (cm), and weight (kg). Body mass index (BMI) was calculated by dividing the patients’ weight by the square of their length or height (kg/m2). The percentile curves for height, weight, and BMI, as well as the height velocity curves, were plotted on the MPS IVA growth charts determined by previous studies [1, 4, 16].
Results
Study population
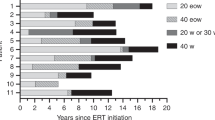
Sixty-seven MPS IVA patients with ERT comprised 36 males and 31 females, and 61 untreated MPS IVA patients comprised 24 males and 37 females. The mean duration of ERT for males and females was 3.17 ± 2.0 and 3.07 ± 1.8 years, respectively. The shortest and longest ERT durations were 0.5 and 6.6 years, respectively. Phenotypes were determined by the height; patients above the 75th percentile on the MPS IVA growth chart for each gender were classified as attenuated, and those beneath the 75th percentile were classified as severe [1]. Twenty-one patients corresponded to the attenuated form of the disease, 101 patients had severe phenotype, and 6 patients had unknown phenotypes due to lack of data or because too young to determine their phenotype. Data were categorized into three age groups: under 5 years of age, between 5 and 10 years of age, and over 10 years of age, to determine the efficiency of ERT with age. All anthropometric measures were classified into two sets to compare untreated and treated groups; one from untreated patients or before ERT, and the other from treated patients.
Length and height
Patients who started ERT under 5 years of age
The mean birth lengths for affected boys and girls were 52.21 ± 3.5 cm (n = 41; n represents the number of data points) and 52.05 ± 3.1 cm (n = 32), respectively, which were similar to those of boys and girls from the MPS IVA growth chart [1] (Table 2).
There were 14 (eight males and six females) patients who started ERT under 5 years of age. Five male and five female patients received ERT before 3 years of age, and three male patients and one female patient started ERT between 3 and 5 years of age. Twelve of 14 patients (six males, six females) received ERT for at least 2 years and were analyzed further (Table 1). One 7-year-old female patient discontinued ERT after 2 years of treatment (between 2 and 4 years old) because of a lack of effectiveness in skeletal manifestations.
The mean height of both treated and untreated boys and girls was slightly higher than that of the normal population at 1 year of age; however, after this age, the mean height of both treated and untreated boys and girls began to fall markedly below that of the normal population (Fig. 1; Supplemental Information Fig. 3) similar to that in Morquio A growth chart [1].
None of 12 patients who received ERT over 2 years experienced a significant height increase. Six out of the 12 patients stopped growing between 5.0 and 9.0 years. The other six patients except one attenuated case had a marked slow growth velocity at the end of this study (Table 1; see “Height velocity” section).
One female patient (Case 1) saw a significant increase in height at the beginning of treatment. This patient was 67.0 cm at 2.0 years of age (beneath the third percentile for height) and reached 94 cm by 4.5 years (between the 50th and 75th percentile); however, the patient stopped growing by 4.5 years, with a final height of 94 cm at 5.8 years (10th–25th percentile) (Fig. 1 and Table 1). The five patients (two males and three females) who started ERT before 3 years of age and continued treatment beyond 5 years old were followed up (Table 1). Two patients (Cases 5 and 6) remained smaller than age-matched MPS IVA patients. An attenuated female patient (Case 11) exhibited a large increase in height; however, she grew 2.0 cm during the last one year with slow growth velocity (see below at “Height velocity” section). The four patients treated between 3 and 5 years old (three males and one female) were followed up beyond 5 years (Table 1). Only one male attenuated patient obtained a significant height increase (Case 7); however, his growth was consistent with that of untreated male attenuated patients. Two patients had heights consistent with that of the age-matched MPS IVA patients (Cases 2 and 3). One male patient remained much smaller than age-matched MPS IVA patients (Case 8). All of the five patients who received ERT before 3 years old stopped growing between the ages of 5.0 and 9.0 years, and one patient treated at 4.5 years old stopped growing at 9.0 years of age.
Patients who started ERT between 5 and 10 years of age
There were three male and four female patients who started ERT treatment between 5 and 10 years of age.
All treated patients with the severe phenotype in this age group exhibited height consistent with or lower than the age-matched height from the MPS IVA growth chart. The mean heights of both treated and untreated patients were similar (Table 2). Except for the continual growth of attenuated patients, the heights of all affected males and females plateaued as similarly observed in the untreated patients. Overall, treated patients in this age group did not experience an increase in growth beyond that of untreated patients.
Patients who started ERT over 10 years of age
There were 21 males and 17 females who started ERT over 10 years of age. None of the treated patients in this age group experienced any significant increase in height (Table 2, Fig. 1). The mean height for treated males and females at 18 years of age was 114.7 ± 17.8 cm (n = 13; between the 25th and 50th percentile) and 119.5 ± 20.5 cm (n = 11; between the 75th and 90th percentile), respectively, corresponding to a −7.9SD for males and a −6.7SD for females on the CDC growth chart. The mean height for untreated males and females at 18 years of age was 110.2 ± 19.2 cm (n = 15; between the 25th and 50th percentile) and 112.4 ± 21.4 cm (n = 26; between the 50th and 75th percentile), corresponding to −8.5SD and −7.8SD for males and females on the CDC growth chart. The large mean height for treated females was attributed to a small data set and a high number of attenuated females. The mean heights of MPS IVA males and females from the reported growth chart at 18 years of age were 119.3 ± 22.6 and 113.5 ± 23.1 cm, respectively [1].
Overall, untreated males stopped growing at a mean age of 9.90 years with a mean height of 99.1 ± 7.71 cm, whereas treated males ceased growing at a mean age of 10.3 years with a mean height of 100.6 ± 6.74 cm. Untreated females stopped growing at a mean age of 9.63 years with a mean height of 99.3 ± 10.7 cm, whereas treated females ceased growing at a mean age of 10.6 years and with a mean height of 100.6 ± 5.70 cm.
Height velocity
Patients who started ERT under 5 years of age
Excluding the female patient (Case 4) who discontinued ERT, 11 patients started ERT under 5 years old. Seven patients starting ERT before 3 years of age (three males, four females) were followed up for growth velocity over 2 years (Table 1; Fig. 2).
-
1.
The female patient (Case 1) had a significantly higher height velocity (12.7 cm/year) at 2.5 years than age-matched MPS IVA females; however, her height stopped by 4.5 years of age.
-
2.
The male patient (Case 5) had a height velocity of 1.76 cm/year at 2.8 years of age, 1.3 years after treatment, indicating a significantly lower height velocity at this age. His growth velocity stopped by 5.3 years.
-
3.
The female patient (Case 6) displayed a declining height velocity from 4.25 cm/year at 4.9 years of age to 1.33 cm/year at 5.3 years of age. This patient exhibited a decrease in height velocity at 5.9 years of age with a value of 0.50 cm/year and showed a peak in height velocity at 6.7 years of age at 2.40 cm/year, consistent with the age-matched untreated patients. Her growth ceased at 7.2 years.
-
4.
The female patient (Case 9) experienced a large peak in height velocity (7.0 cm/year) at 2.8 years of age, which was equivalent to normal females; however, her growth velocity declined to 2.4 cm/year at 3.1 years of age, similar to the age-matched untreated females.
-
5.
The male patient (Case 10) had a higher height velocity (5.17 cm/year) than the age-matched patients at 3.75 years old. The patient’s height velocity then declined to 2.99 cm/year at 4.33 years old, similar to that of the age-matched untreated males.
-
6.
The female patient (Case 11) experienced higher height velocity (5.0 cm/year) than age-matched MPS IVA females at 3.4 years. She exhibited a slightly declining height velocity of 2.50 cm/year at 5.3 and 5.9 years of age. Her growth velocity decreased further at 6.9 years (2.0 cm/year) below the average of age-matched untreated females.
-
7.
The male patient (Case 12) had a higher height velocity (5.6 cm/year) than the age-matched untreated patients at 2.5 years; however, his height velocity declined to 2.35 cm/year one year after treatment, indicating a lower height velocity than the age-matched MPS IVA males. The patient had a peak in height velocity of 5.00 cm/year between 5.2 and 5.4 years of age; however, the patient stopped growing at 5.6 years.
Height velocity (cm/year) of MPS IVA males and females who started ERT under 10 years old. The black points indicate prior or after ERT treatment and the red points indicate ERT treatment. The blue line shows the 50th percentile for height velocity for healthy males and females. The gray line shows the 50th percentile for height velocity for MPS IV males and females
Four patients starting between 3 and 5 years of age were followed up for growth velocity (Table 1; Fig. 2).
(1) The female patient (Case 2) had height velocities of 2.40 and 2.43 cm/year at 5.3 and 5.8 years of age, consistent with the height velocities of untreated MPS IVA females.
(2) The male patient (Case 3) exhibited a decline in height velocity from 5.71 cm/year at 4.2–2.00 cm/year at 6.3 years of age. This patient displayed a height velocity of 2.86 cm/year at 6.8 years of age, consistent with the age-matched untreated patients.
3) The attenuated male patient (Case 7) displayed a large peak in height velocity, with a value of 5.89 cm/year at 5.2 years old and 3.75 cm/year at 6.1 years old. This patient displayed a peak in height velocity one year earlier than the age-matched patients.
4) The male patient (Case 8) exhibited a decline in height velocity to 1.00 cm/year at 6.6 years of age. The patient displayed a peak in height velocity of 2.78 cm/year at 8.1 years of age, consistent with the height velocity of untreated MPS IVA males; however, he stopped growing at 9.0 years.
Both males and females treated before 3 years of age and those treated between 3 and 5 years of age had height velocities similar to those of age-matched untreated patients (Table 3; Supplemental Information Fig. 4).
Patients who started ERT between 5 and 10 years of age
Four male and four female patients started ERT between 5 and 10 years of age. All four male patients were treated for over 2.5 years, and four female patients were treated for over 4.5 years. Treated male patients had height velocities similar to age-matched untreated patients. Treated males displayed reduced height velocity at 9.0 years of age, which reflected a similar trend seen in age-matched untreated males in the MPS IVA growth chart [1] (Fig. 2). Treated female patients primarily exhibited height velocities consistent with those of the female MPS IVA growth chart. Peaks in height velocity of treated female patients were observed at 3 and 5 years of age, whereas rapid declines in height velocity were exhibited at 4 and 8 years of age (Fig. 2).
In spite of the continual growth in both treated and untreated attenuated patients, their height velocities declined with age (Table 3). Treated males and females did not experience a significant increase in height velocity as compared to untreated males and females (Table 3; Fig. 2; Supplemental Information Fig. 4).
Patients who started ERT over 10 years of age
Eleven male and 11 female patients started ERT after 10 years of age. The mean height velocities of treated and untreated males and females did not significantly differ (Table 3). Both treated and untreated MPS IVA males and females over 10 years of age exhibited reduced growth spurts, compared with the normal population (Supplemental Information Fig. 4).
Treated and untreated males exhibited a slightly accelerated peak at 8.0 and 10.5 years of age, as previously seen in the MPS IVA growth chart [1]. Untreated males also experienced a peak at 12 years old. Rapid declines in height velocity of treated males were observed at 12 years old. Treated and untreated females exhibited an accelerated peak in height velocity between 11 and 12 years of age, similar to that of females on the MPS IVA growth chart. Conversely, they displayed a sharp decline in height velocity at 13 years of age. The same trend was seen in females from the MPS IVA growth chart [1].
Our study showed that both treated and untreated males and females exhibited reduced pubertal growth spurts as compared to age-matched normal controls. Treated patients did not experience a significant increase in height velocity as compared to untreated males and females.
Weight and BMI
The mean birth weights for males and females were 3.57 ± 0.59 kg (n = 42) and 3.48 ± 0.63 kg (n = 36), which was similar to those of males and females on the MPS IVA growth chart (3.56 kg and 3.50 kg, respectively) [1]. The mean birth weight for healthy males and females was 3.53 and 3.40 kg, as found on the CDC growth chart, thus, the birth weight of MPS IVA patients in this study was heavier than that of normal controls. The weights of both treated and untreated patients were similar to that of the normal population until 4 years of age, and remained analogous to patients from the MPS IVA growth chart (Supplemental Information Figs. 1 and 5). There was no significant difference in the weights of treated and untreated patients across all age groups (Supplemental Information Table 1). The mean weight for untreated males and females at 18 years of age was 32.48 ± 13.9 kg (n = 18) and 31.78 ± 9.0 kg (n = 28), respectively, corresponding to −6.8 SD and −6.3 SD of the weight for age-matched normal males and females. The mean weight for treated males and females at 18 years of age was 37.91 ± 13.2 kg (n = 12) and 35.30 ± 11.4 kg (n = 14), corresponding to a −4.9 SD and −4.6 SD of the weight for healthy age-matched males and females. Thus, the mean weight for treated patients at 18 years of age was significantly higher than that of untreated patients (Supplemental Information Table 1).
An upward shift of the mean BMI was observed in both treated and untreated patients across all age groups compared with healthy age-matched controls (Supplemental Information Figs. 2 and 6). The mean BMI for untreated males and females at 18 years of age was 26.45 ± 5.0 and 25.25 ± 4.5 kg/m2, respectively, corresponding to +1.2SD and +0.9SD of the BMI for age-matched normal males and females. The mean BMI for treated males and females at 18 years of age was 28.14 ± 6.3 and 24.73 ± 3.1 kg/m2, corresponding to +1.5SD and +0.8SD of the BMI for healthy age-matched controls (Supplemental Information Table 2). BMI above the 95th percentile (34.7 and 34.4 kg/m2 for males and females with MPS IVA [1]; indicating being obese) was observed in 5.0% of males (n = 3; one treated, two untreated) and 2.9% of females (n = 2; one treated, one untreated) with MPS IVA aged 18 years and older. All of the patients classified as obese started ERT over 10 years of age. BMI above the 85th percentile and less than the 95th percentile (31.9 and 32.4 kg/m2 for males and females with MPS IVA [1], indicating being overweight) was observed in 3.3% of males (n = 2) and 1.5% of females (n = 1) with MPS IVA aged 18 years and older (Supplemental Information Figs. 2 and 6). All of the patients that were overweight were untreated. As both treated and untreated patients had similar BMI’s and tendencies of being obese and overweight, our study indicates that ERT does not affect BMI.
Discussion
In this study, we have demonstrated (1) that MPS IVA males and females treated with ERT before 5 years of age failed to show growth improvement, (2) that treated and untreated patients with MPS IVA reached their final heights at ~10 years of age, (3) that MPS IVA patients treated with ERT had a reduced pubertal growth spurt similar to untreated patients, (4) that patients treated with ERT did not show any increase in growth apart from natural growth in any of the age groups, and (5) that the proportion of obese and overweight patients was similar in treated and untreated patients.
In our evaluation of patients starting ERT under 5 years of age, we found that patients did not show improvement in growth measurements evaluated. Six out of 12 patients (50%) treated with ERT before 5 years of age for over 2 years stopped growing before 10 years of age, and four patients stopped growing at 5 years old. Five of those six patients who stopped growing received ERT before 3 years old, indicating that ERT is not effective in bone growth even when administered before 3 years of age.
We found that all treated patients who received ERT under 5 years of age exhibited height consistent with the average MPS IVA male and female, or even less than average heights of age-matched untreated patients. Patients starting ERT under 5 years old also had a rapid decline in height velocity as seen in untreated patients. Patients who received ERT before 5 years of age also displayed similar weight and BMI trends as untreated patients with the same phenotype. Taken together, our results indicate that ERT did not improve the growth of patients who started under 5 years of age, as shown by the continued marked short stature, compared to the age-matched control population.
There were several studies described for the growth in MPS IVA patients with ERT [36, 43,44,45]. In 2015, Hendriksz et al. described little impact on growth in patients over 5 years of age in phase III trial [45] (Supplemental Information Table 3). In 2015, Jones et al. described the growth in MPS IVA patients under 5 years of age with one year of ERT, suggesting that there is a trend toward improvement in growth without clear proof and that a long-term observation is required [43, 44]. These did not use the natural Morquio growth chart in comparison. In 2016, Cao et al. indicated that early ERT starting at 21 months did not improve the bone outcome in a severe MPS IVA patient, as determined after the 30 month-long treatment [36]. The height of this patient increased during the first year of the ERT, but no more height gain was observed thereafter for 18 months. Bone deformities (including severe pectus carinatum) worsened, and his medullar cervical spine compression showed no improvement, requiring decompression surgery. The findings in our study were compatible with this result.
Our study has shown that newborn length and weight for both males and females were similar or slightly increased, compared to those of the normal population. This finding is similar to previous reports, suggesting that the diagnosis of MPS IVA at birth proves difficult unless bone deformities associated with skeletal dysplasia are present [1, 4]. We observed that the growth patterns of both treated and untreated MPS IVA patients are characterized by impaired growth velocity after one year of age and that both treated and untreated patients reach their final heights by ~10 years of age. Comparing patients treated with ERT before 5 years of age, between 5 and 10 years of age, and after 10 years of age, we found that there was no significant difference in height velocity between treated and untreated patients in all age groups. Thus, our study indicates that current ERT is not effective in improving the bone growth of MPS IVA patients, even when administered before 5 years of age; however, more studies following patients with ERT especially before 2 years would be required to confirm these findings.
Our study also found that both treated and untreated MPS IVA patients had a reduced pubertal growth spurt, which contributes to marked short stature. It has been found that the growth plate is severely affected and destroyed before the pubertal growth spurt, which leads to growth impairment [1, 17]. Reduced pubertal growth spurt findings were consistent with previous studies [1].
Poor growth is equated with poor health in pediatric practice, and poor childhood growth leads to the risk of adult hip fracture [4, 46]. Poor growth can be evaluated through careful measurement and comparison with appropriate reference standards [4]. Thus, it is important to monitor and understand the growth of MPS IVA patients and compare these measurements to MPS IVA growth charts, as well as CDC growth charts for the normal population. Since MPS IVA is a rare disorder, limited data exists from each age group, providing a limitation in our study. Thus, the growth data that we collected from MPS IVA patients investigated here could have been slightly skewed by lack of data when compared with growth charts of the previous MPS IVA patients [1, 4]. Despite the limitation of data, comparison of our data with that from MPS IVA growth charts and the CDC growth charts has led us to conclude that severe growth impairment in children with MPS IVA occurs after 1 year of age.
The mean weights of MPS IVA patients were considerably lower across all age groups examined when compared with normal age-matched controls; however, due to their short stature, 5.0% of males and 2.9% of females were obese in the MPS IVA population. In addition, 3.3% of males and 1.5% of females were overweight. The proportion of obesity and overweight was similar in both treated and untreated patients, and thus ERT itself did not affect obesity in MPS IVA patients in this study. This is unexpected since the ADL correlates reversely with obesity. It is of great interest to know whether the treated patients here in this study have increased ADL or not. When compared with the normal population, there was an upward shift of the mean BMI in both treated and untreated MPS IVA patients across all age groups examined. Thus, MPS IVA patients are at a greater risk of obesity and being overweight than the normal population. In addition to the risk presented by obesity to the general population, stress on susceptible bones and joints is caused by being overweight in those with severe bone dysplasia, resulting in a wheel-chair bound condition and successive neurological and orthopedic complications [4]. Thus, MPS IVA patients with marked short stature should avoid obesity [1, 16].
While there is currently no medication to prevent or cure MPS IVA, ERT was approved for the treatment of the disorder [22, 23]. A reduction of urinary KS and an improvement in the 6-min walk test (6MWT) were observed in studies regarding ERT (but no improvement of 6MWT in an extension study); however, it has been shown that the enzyme used for ERT has a short half-life and is rapidly cleared from circulation [23, 32], the cost of treatment is high [22, 23], the enzyme barely reaches bone [28], KS in blood is not reduced in parallel with urinary KS reduction [47], and surgical remnants during 6–60 months of ERT (5–24 years old) do not show any clearance of storage materials in chondrocytes [22, 29]. Our study indicated that MPS IVA patients treated with ERT did not show significant growth apart from natural growth across all age groups examined. This finding is consistent with previous studies predicting that intravenous ERT provides very limited impact on the bone pathology of MPS IVA in humans and murine [22, 23, 32]. From our study, we can infer that as MPS IVA is primarily a skeletal disorder, ERT does not prove effective even when administered under 5 years of age.
Overall, our manuscript includes three novel findings: (1) we have proved no impact on the growth by ERT with comprehensive data on growth effects, (2) we have confirmed the final height in 6 of 12 patients under 5 years with the follow-up of at least over 2 years after starting of ERT, and (3) we have used the Morquio A growth chart as a natural history in comparison which provides a more precise assessment of the growth impact.
Further studies with larger sample sizes and/or patients treated under 2 years of age are needed to evaluate whether intravenous ERT can improve the growth of MPS IVA patients. In conclusion, we have found that both treated and untreated children with MPS IVA exhibit poor growth and that treated patients do not experience growth improvement.
References
Tomatsu S, Montaño AM, Oikawa H, Giugliani R, Harmatz P, Smith M, et al. Impairment of body growth in mucopolysaccharidoses. In: Preedy V. (ed.) Handbook of growth and growth monitoring in health and disease. New York, NY: Springer; 2012. p. 2091–117.
Doherty C, Averill LW, Theroux M, Mackenzie WG, Pizarro C, Mason RW, et al. Natural history of Morquio A patient with tracheal obstruction from birth to death. Mol Genet Metab Rep. 2018;14:59–67.
Tomatsu S, Montaño AM, Lopez P, Trandafirescu G, Gutierrez MA, Oikawa H, et al. Determinant factors of spectrum of missense variants in mucopolysaccharidosis IVA gene. Mol Genet Metab. 2006;89:139–49.
Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet Part A. 2008;146:1286–95.
Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–74.
Dũng VC, Tomatsu S, Montaño AM, Gottesman G, Bober MB, Mackenzie W, et al. Mucopolysaccharidosis IVA: correlation between genotype, phenotype and keratan sulfate levels. Mol Genet Metab. 2013;110:129–38.
Khan S, Alméciga-Díaz CJ, Sawamoto K, Mackenzie WG, Theroux MC, Pizarro C, et al. Mucopolysaccharidosis IVA and glycosaminoglycans. Mol Genet Metab. 2017;120:78–95.
Tomatsu S. Mucopolysaccharidosis Type IVA: clinical features, biochemistry, diagnosis, genetics, and treatment. Mucopolysaccharidoses update: (2 volume set) Ch. 12. New York: Nova Science Publishers; 2018. p. 235–71.
Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997 101:355–8.
Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105:e10.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54.
Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–6.
Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12:87–92.
Khan S, Orii T, Giugliani R, et al. Epidemiology of mucopolysaccharidoses. In: Shunji Tomatsu, Christine Lavery, Roberto Giugliani, Paul Harmatz, Maurizio Scarpa, Grzegorz Węgrzyn and Tadao Orii (eds.) Mucopolysaccharidoses update, vol. 1, Ch. 12. New York: Nova Science Publishers; 2018. p. 21–45.
Khan SA, Peracha H, Ballhausen D, Wiesbauer A, Rohrbach M, Gautschi M, et al. Epidemiology of mucopolysaccharidoses. Mol Genet Metab. 2017;121:227–40.
Melbouci M, Mason RW, Suzuki Y, Fukao T, Orii T, Tomatsu S. Growth impairment in mucopolysaccharidoses. Mol Genet Metab. 2018;124:1–10.
Hendriksz CJ, Harmatz P, Beck M, Jones S, Wood T, Lachman R, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110:54–64.
Peracha H, Sawamoto K, Averill L, Kecskemethy H, Theroux M, Thacker M, et al. Molecular genetics and metabolism, special edition: diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA. Mol Genet Metab. 2018;125:18–37.
Tomatsu S, Averill LW, Sawamoto K, Mackenzie WG, Bober MB, Pizarro C, et al. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol Genet Metab. 2016;117:150–6.
Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation (Case Presentation). Acta Paediatr. 2009;98:768–9.
Tomatsu S, Montaño AM, Oikawa H, Smith M, Barrera L, Chinen Y, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–45.
Tomatsu S, Giugliani R, Kubaski F, Kazuki S, Alméciga-Díaz CJ, Barrera L, et al. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des Dev Ther. 2015;9:1937.
Tomatsu S, Sawamoto K, Shimada T, Bober MB, Kubaski F, Yasuda E, et al. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations. Expert Opin Orphan Drugs. 2015;3:1279–90.
Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, et al. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol Genet Metab. 2016;117:84–94.
Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41.
Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, et al. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2014;37:979–90.
Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–77.
Tomatsu S, Montaño AM, Dung VC, Ohashi A, Oikawa H, Oguma T, et al. Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol Ther. 2010;18:1094–102.
Tomatsu S, Alméciga-Díaz CJ, Montaño AM, Yabe H, Tanaka A, Dung VC, et al. Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab. 2015;114:94–109.
Pizarro C, Davies RR, Theroux M, Spurrier EA, Averill LW, Tomatsu S. Surgical reconstruction for severe tracheal obstruction in Morquio A syndrome. Ann Thorac Surg. 2016;102:e329–31.
Tomatsu S, Montano AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2007;17:815–24.
Qi Y, Musson DG, Schweighardt B, Tompkins T, Jesaitis L, Shaywitz AJ, et al. Pharmacokinetic and pharmacodynamic evaluation of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome. Clin Pharm. 2014;53:1137–47.
Rohrbach M, Clarke JTR. Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs. 2007;67:2697–716.
Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al. A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1. Health Technol Assess. 2006;10:iii–iv, ix–113.
Puckett Y, Mulinder H, Montaño AM. Enzyme replacement therapy with elosulfase alfa for mucopolysaccharidosis IVA (Morquio A syndrome): milestones and challenges. Expert Opin Orphan Drugs. 2017;5:741–52.
Do Cao J, Wiedemann A, Quinaux T, Battaglia-Hsu SF, Mainard L, Froissart R, et al. 30 months follow-up of an early enzyme replacement therapy in a severe Morquio A patient: about one case. Mol Genet Metab Rep. 2016;9:42–5.
Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–8.
Montaño AM, Oikawa H, Tomatsu S, Nishioka T, Vogler C, Gutierrez MA, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab. 2008;94:178–89.
Nishioka T, Tomatsu S, Gutierrez MA, Miyamoto K, Trandafirescu GG, Lopez PLC, et al. Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide. Mol Genet Metab. 2006;88:244–55.
Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, et al. Novel drug delivery system to bone using acidic oligopeptide: pharmacokinetic characteristics and pharmacological potential. J Drug Target. 2001;9:111–21.
Yasuda E, Suzuki Y, Shimada T, Sawamoto K, Mackenzie WG, Theroux MC, et al. Activity of daily living for Morquio A syndrome. Mol Genet Metab. 2016;118:111–22.
Nagao K, Morlet T, Haley E, Padilla J, Nemith J, Mason RW, et al. Neurophysiology of hearing in patients with mucopolysaccharidosis type IV. Mol Genet Metab. 2018;123:472–8.
Jones SA, Bialer M, Parini R, Martin K, Wang H, Yang K, et al. Safety and clinical activity of elosulfase alfa in pediatric patients with Morquio A syndrome (mucopolysaccharidosis IVA) less than 5 y. Pediatr Res. 2015;78:717–22.
Concolino D, Deodato F, Parini R. Enzyme replacement therapy: efficacy and limitations. Ital J Pediatr. 2018;44(S2):120.
Hendriksz CJ, Giugliani R, Harmatz P, Mengel E, Guffon N, Valayannopoulos V, et al. Multi-domain impact of elosulfase alfa in Morquio A syndrome in the pivotal phase III trial. Mol Genet Metab. 2015;114:178–85.
Oliver H, Jameson KA, Sayer AA, Cooper C, Dennison EM, Hertfordshire Cohort Study Group the HCS. Growth in early life predicts bone strength in late adulthood: the Hertfordshire Cohort Study. Bone. 2007;41:400–5.
Khan SA, Mason RW, Giugliani R, Orii K, Fukao T, Suzuki Y, et al. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol Genet Metab. 2018;125:44–52.
Acknowledgements
This work was supported by grants from The Carol Ann Foundation, Angelo R. Cali & Mary V. Cali Family Foundation, Inc., The Vain and Harry Fish Foundation, Inc., The Bennett Foundation, Jacob Randall Foundation, Austrian and Japanese MPS societies, and Nemours Funds. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of National Institutes of Health (NIH) under grant number P30GM114736. The content of the article has not been influenced by the sponsors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Doherty, C., Stapleton, M., Piechnik, M. et al. Effect of enzyme replacement therapy on the growth of patients with Morquio A. J Hum Genet 64, 625–635 (2019). https://doi.org/10.1038/s10038-019-0604-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0604-6
This article is cited by
-
Morquio A syndrome and effect of enzyme replacement therapy in different age groups of Turkish patients: a case series
Orphanet Journal of Rare Diseases (2021)
-
Elosulfase alfa in the treatment of mucopolysaccharidosis type IVA: insights from the first managed access agreement
Orphanet Journal of Rare Diseases (2021)
-
Treatment of skeletal and non-skeletal alterations of Mucopolysaccharidosis type IVA by AAV-mediated gene therapy
Nature Communications (2021)
-
Bone-Specific Drug Delivery for Osteoporosis and Rare Skeletal Disorders
Current Osteoporosis Reports (2020)
-
Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future
Journal of Human Genetics (2019)