Abstract
Background:
Continuous positive airway pressure (CPAP) is a primary form of respiratory support used in the intensive care of preterm infants, but its long-term effects on airway (AW) function are unknown.
Methods:
We developed a neonatal mouse model of CPAP treatment to determine whether it modifies later AW reactivity. Unanesthetized spontaneously breathing mice were fitted with a mask to deliver CPAP (6 cmH2O, 3 h/day) for 7 consecutive days starting at postnatal day 1. AW reactivity to methacholine was assessed using the in vitro living lung slice preparation.
Results:
One week of CPAP increased AW responsiveness to methacholine in male, but not female mice, compared to untreated control animals. The AW hyper-reactivity of male mice persisted for 2 wk (at P21) after CPAP treatment ended. Four days of CPAP, however, did not significantly increase AW reactivity. Females also exhibited AW hyper-reactivity at P21, suggesting a delayed response to early (7 d) CPAP treatment. The effects of 7 d of CPAP on hyper-reactivity to methacholine were unique to smaller AWs whereas larger ones were relatively unaffected.
Conclusion:
These data may be important to our understanding of the potential long-term consequences of neonatal CPAP therapy used in the intensive care of preterm infants.
Similar content being viewed by others
Main
Airway (AW) hyper-reactivity associated with asthma and wheezing disorders represent major long-term respiratory morbidities of former preterm infants (1,2,3). Supplemental O2 and continuous positive AW pressure (CPAP) have become the primary modes of respiratory support for preterm infants with respiratory distress syndrome (4,5). It has become widely accepted that supplemental O2 therapy (among other factors) is likely a major contributor to the pathogenesis of AW hyper-reactivity, which has been corroborated by numerous animal studies (6,7,8,9,10). However, virtually nothing is known about the long-term consequences neonatal CPAP (with or without O2 exposure) could have on the etiology of AW reactivity (11). For the purpose of the current study, we developed a novel mouse model to test the hypothesis that neonatal CPAP alone (without hyperoxia) could cause a long-term increase in AW reactivity.
Data from animal models used to investigate the effects of neonatal supplemental O2 therapy support the clinical findings of increased lung inflammation (7,12), remodeling including alveolar simplification, and smooth muscle proliferation (10,11,13,14). The latter would in part explain the associated increase in AW reactivity. Clinically, supplemental O2 and CPAP, however, are often used together, making it difficult to delineate how either intervention alone could contribute to physiological changes in lung development and pulmonary function.
In recent years, there has been a shift in neonatal intensive care unit (NICU) practice from the use of intubation and mechanical ventilation toward less invasive CPAP treatment, largely because of the baro- or volu-trauma imposed on the lung by the former mode of respiratory support (15,16). Indeed, CPAP has the potential to mitigate the adverse pathophysiological effects on lung development that would otherwise be observed with mechanical ventilation. However, there are no animal studies that have investigated the effects of neonatal CPAP on AW reactivity, and the limited studies done on adults have yielded conflicting results. In adult humans with asthma (17) or sleep apnea (18), CPAP reduced AW reactivity while others have shown CPAP caused it in sleep apnea patients (19,20). In 7–8-wk-old unanesthetized ferrets, continuous (24 h/day, 4 d) or nocturnal (12 h/day) CPAP reduced AW responses to acetylcholine (21). Collectively, these data suggest the effects of CPAP on AW reactivity are complex and likely reflect several factors including duration, intensity and the experimental setting used to deliver pressure to the lung, as well as developmental influences. There currently is no small (e.g., rodent) neonatal animal model of CPAP available because of the major challenges associated with delivering it noninvasively. Recently, we have developed an animal model in which CPAP can be successfully administered daily and noninvasively to unanesthetized neonatal mice for the first week of postnatal life. In this study, we tested the hypothesis that daily neonatal CPAP would elicit a long-lasting increase in AW reactivity. Further, given the sex differences in the prevalence of wheezing disorders (22) and AW hyper-reactivity (23), we also investigated whether the effects of CPAP would be different between male and female mice.
Results
P8 AW Reactivity to Methacholine Challenge
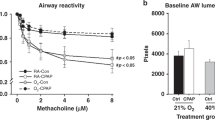
AW responses to bath-applied methacholine challenge for ~8-d-old male and female mice are provided in Figure 1 . The slope of the small (baseline AW lumen area <4,000 pixels) AW response to increasing doses of methacholine was greater in 8-d-old male mice following 7 d of neonatal CPAP treatment compared to control mice, as indicated by the larger decrease in AW luminal area at higher doses of methacholine ( Figure 1a , P < 0.05). However, reactivity of larger AWs (baseline AW lumen area >4,000 pixels) to methacholine was not affected by CPAP ( Figure 1b ). In contrast to male mice, neither small nor large AWs of female mice were affected by neonatal CPAP ( Figure 1c , d ). These data suggest that males are more sensitive to neonatal CPAP than females during the first week of life, and these effects are limited to the smaller AWs.
Airway (AW) responses to methacholine challenge in the in vitro living lung slice preparation from ~8-d-old (a,b) male and (c,d) female mice that received 7 d of neonatal (P1–7) continuous positive airway pressure (CPAP) (6 cmH2O). CPAP (square symbols) increased small AW (a) responses to methacholine in males compared to Ctrl mice (circle symbols), whereas larger AWs (b) were not affected by CPAP. In contrast, neither the small (c) or large (d) AWs of female mice were affected by CPAP. Values in a and c are “small” AWs defined as a baseline lumen area <4,000 pixels; “large” AWs (b and d) defined as baseline lumen area >4,000. b and d are the response to maximum (8 μmol/l) methacholine. *indicates significant difference in the slope of the response between Ctrl and CPAP animals (N = 7–10 airways from four to five mice/group; P < 0.05).
P21 AW Reactivity to Methacholine Challenge
AW reactivity to methacholine in 3-wk-old mice as assessed ~2 wk after CPAP treatment ended is provided in Figure 2 . Compared to control mice, 7 d of CPAP caused an increase in AW constriction to bath-applied methacholine in both male ( Figure 2a ) and female ( Figure 2c ) mice, as indicated by the larger decrease in AW luminal area. The augmented AW reactivity following CPAP was limited to the smaller AWs (<7,000 pixels) for both male and female mice, whereas larger AWs (>7,000) were relatively unaffected ( Figure 2b , d ). Further, 4 d of CPAP treatment (between P1–4) also had a tendency to increase AW reactivity to methacholine in the small AWs, although this did not reach statistical significance from ctrl mice ( Figure 2 ). These data suggest the effects of 7 d of CPAP on smaller AWs that occurred at P8 in the males (see Figure 1a ) persisted for 2 wk after CPAP treatment ended. Interestingly, CPAP appeared to elicit a delayed effect on AW reactivity of female mice until 2 wk after CPAP treatment. In fact, the magnitude of CPAP-induced AW hyper-reactivity at P21 was similar between males and females ( Figure 3a ) and, compared to respective control mice, both sexes were equally responsive to methacholine following CPAP ( Figure 3b ).
Airway (AW) responses to methacholine challenge in the in vitro living lung slice preparation from 21-d-old (a,b) male and (c,d) female mice that received either 4 (triangles) or 7 (squares) days of neonatal continuous positive airway pressure (CPAP) (6 cmH2O). Note, 7 d of CPAP increased AW reactivity in small AWs of both male (a) and female (c) mice compared to control (Ctrl, circles) animals. The large AWs of both male (b) and female (d) mice were not affected by CPAP. “Small” AWs (a and c) are defined as baseline lumen area <7,000 pixels; “large” AWs (b and d) are defined as baseline lumen area >7,000 at baseline. *indicates significant difference in the slope of the response to methacholine of Ctrl animals (N = 6–8 airways from four to five mice/group); #indicates significant difference in the slope of the response to 4 d of CPAP (P < 0.05).
Small Airway (AW) (<7,000 pixel baseline lumen area) responses to methacholine challenge in (a) 21-d-old male (black symbols) and female (open symbols) control mice and (b) the effects of 7 d of neonatal continuous positive airway pressure (CPAP) on small AW responses to maximum (8 μmol/l) dose of methacholine. AW reactivity to methacholine was similar between male and female control mice (a) and both sexes were equally affected by 7 d of neonatal CPAP (b). Values in a are expressed as a fraction of baseline lumen area; whereas values in b are expressed as absolute AW area at maximum contraction (8 μmol/l, methacholine). Inset: average values for the mean AW size (at baseline) for both male and female mice was not different. *indicates significant slope of the response to methacholine (P < 0.05); however, there was no difference in the slopes between sexes, suggesting males and females are equally sensitive to CPAP treatment.
Lung Morphology
To assess the long-term effects of 7 d of CPAP treatment, we quantified the radial alveolar counts in P21 mice. There was a subtle (~10%), but significant reduction in radial alveolar counts at P21, 2 wk after CPAP treatment ended compared age-matched control treated mice ( Figure 4 ). Representative lung sections from ctrl and CPAP-treated mice are also provided ( Figure 4 ).
Average (a) radial alveolar counts from 21-d-old male that received 7 d of continuous positive airway pressure (CPAP) (6 cmH2O); and (b) representative images of a lung from a control (top) and CPAP (bottom) mouse. Note, neonatal CPAP treatment caused a subtle (10%), yet significant reduction in radial alveolar counts compared to Ctrl mice. *significantly different from Ctrl mice (P < 0.05). Scale: 100 μm.
Discussion
The principal findings of the current study demonstrated: (i) daily neonatal CPAP (7 d) increased AW reactivity in male mice; (ii) augmented AW reactivity persisted for 2 wk after CPAP treatment ended; (iii) development of AW hyper-reactivity was delayed in females; and (iv) the effects of CPAP were restricted to the smaller AWs. We used the living lung slice preparation to assess AW responses to methacholine because it is a convenient technique that circumvents the technical challenges associated with in vivo measurements of respiratory mechanics in very small neonates. This technique also has the advantage of allowing direct visualization of individual AWs of various sizes.
The lack of a convenient animal model has hindered our progress in understanding how CPAP could contribute to the long-term development of AW reactivity commonly seen among preterm infants. Indeed, there are major challenges associated with delivering CPAP or providing lung inflation noninvasively to neonatal animals (24,25,26,27), especially for extended periods of time (days-weeks). These prior studies have contributed substantially to our understanding of how in vivo lung stretch can influence pulmonary function. For the purpose of the current study, we developed a novel rodent model of CPAP which could be successfully delivered daily to neonatal mice. We have shown that even a short (1 wk) period of acute (3 h/day) neonatal CPAP can profoundly augment AW reactivity to methacholine challenge, and it persisted, at least in males, well after (2 wk) CPAP treatment ended. However, a shorter duration of CPAP treatment (4 d) failed to significantly increase AW reactivity indicating a “dose” dependent effect of CPAP. This would be somewhat consistent with neonatal rats that received high intermittent positive pressure (IPP) while mechanically ventilated (25). As little as 6 d of high IPP increased AW reactivity compared to mice treated with low IPP, although the rats were anesthetized daily, paralyzed and mechanically ventilated. Although IPP ventilation is a different stimulus than CPAP used in the current study, these data collectively demonstrate that lung distention during the early neonatal period can cause AW hyper-reactivity.
Few animal studies have investigated the effects of CPAP on neonatal AW reactivity and its effects on adults are conflicting (17,18,19,20,21,28). The discrepancies between these studies suggest the effects of CPAP are multifactorial and could depend on numerous factors including the duration, intensity and experimental setting in which positive pressure was delivered to the lung. Further, it was proposed that AW hyper-reactivity caused by CPAP in adults could have been facilitated by pre-existing pulmonary inflammation (19), which is further supported by an adult rat model whereby CPAP induced lung injury and pulmonary inflammation only when it was administered together with an intravenous injection of lipopolysaccharide (LPS) (29). On the other hand, neonatal data suggest that CPAP may be protective against O2 induced pulmonary inflammation and lung injury and improve alveolar development. In the current study, CPAP also reduced the radial alveolar counts (RAC) which would be consistent with a bronchopulmonary dysplasia (BPD) morphological phenotype; however, O2 exposure has also been shown to cause AW hyper-reactivity and alveolar simplification so it is difficult to predict from the current data what the combined effect of CPAP and O2 might have on the developing lung. Specifically, whether CPAP would elicit beneficial or deleterious effects on lung function could depend on the interaction with other confounding and potentially inflammatory mediating factors. A direct translational comparison between an immature mouse lung and the preterm infant, therefore, should be done with caution as a distending pressure of 6 cmH2O to the smaller, healthy newborn mouse lung may elicit different responses than in the larger, compromised lung of the preterm infant. Future studies should investigate the long-term effects of CPAP in combination with O2 and other clinically relevant scenarios to determine how their interaction might impact pulmonary development and AW reactivity.
The lung of a newborn mouse is at the saccular stage of development (30) and could be considered somewhat comparable to that of an infant born prematurely at 26–28 wk gestation at a time when it is likely to receive CPAP treatment. The developmental immaturity of the P8 mouse lung could explain the lack of a significant response to methacholine ( Figure 1 ). The immature lung, therefore, could be more or less vulnerable to various challenges depending on its stage of development. In support of this, interleukin-1β (IL-1β) arrested alveolar development and caused AW remodeling when overexpressed exclusively during the saccular stage of development (30). These effects were less pronounced if over expression of IL-1β occurred before (the canalicular stage) or after (alveolar stage) this period of lung development, suggesting a unique window of heightened vulnerability to inflammatory insults. Thus, unique characteristics of lung development that occur exclusively during the saccular stage of development may render the lung more vulnerable to certain challenges including CPAP and other modes of lung distention.
The cause of the increased reactivity following neonatal CPAP are beyond the scope of this study. However, we noted the increased AW reactivity of P21 male mice following prior CPAP treatment was associated with a 10% reduction in alveolar counts compared to control mice. The interaction between alveolar simplification and AW hyper-reactivity would be consistent with animal models of BPD that utilize neonatal hyperoxia exposure (10). In fact, the results of the current study demonstrate that neonatal CPAP has a long-term profound effect on AW reactivity independently of hyperoxia exposure which is used routinely in animal models of BPD. A more detailed analysis of pulmonary morphology, particularly related to possible changes in the contractile mechanisms of the AWs are needed. Indeed, studies have shown proliferation and metabolic activity of immature lungs are stimulated by stretch (31) as well as inflammatory cytokine expression (26), increased smooth muscle mass, squamous epithelium metaplasia, (25) and accelerated lung growth (27).
We noted the AW hyper-reactivity following neonatal CPAP was restricted to the smaller AWs, which continued to be observed even 2 wk after CPAP treatment ended, although this effect was unique to males. This is in part consistent with the long-term increase in smooth muscle in the small conducting AWs of 10-mo-old mice exposed to hyperoxia (65% O2) during the first 7 postnatal days (9). The mechanisms determining the selective and persistent effects of CPAP on the smaller AWs remain to be determined, but suggest they may be more vulnerable to lung distention than larger AWs.
Finally, the presence of AW hyper-reactivity in male (but not female) mice at the time of CPAP treatment (P8) could indicate they are more sensitive than females, although it was interesting to note a delayed development of hyper-reactivity in the females. This is surprisingly consistent with the human literature in that preterm boys are at greater risk for BPD (and mechanical ventilation) while in the NICU (32), whereas former preterm girls tended to exhibit a higher prevalence of respiratory complications (asthma and shortness of breath during exercise) at 19 y (22). Although it is difficult to compare a 3-wk-old mouse formerly treated with CPAP with 19-y-old humans, it would be interesting to know how CPAP treatment persists well into adulthood for either male or female mice. Adult female mice exhibited a greater response to inhaled methacholine compared to males when treated with 4 d of hyperoxia from birth (33). Similarly, in normal healthy 6-wk-old male mice, AW reactivity to methacholine was greater than females, although by 12 wk this had reversed as females became more reactive than the males (23). The cause for the delayed development in AW hyper-reactivity in female mice treated with CPAP is beyond the scope of the study, although hormonal influences are likely to play a role. Indeed, resistance to oxygen toxicity was delayed in castrated young male rats (34) whereas estrogen treatment also delayed LPS-induced lung injury and inflammation in ovariectomized female mice (35).
Conclusion
In conclusion, we have developed a novel animal model of neonatal CPAP using an awake, unanesthetized mouse and have demonstrated that a clinically relevant level of CPAP caused a persistent increase in AW reactivity. The neonatal mouse, therefore, may be a promising model to investigate the long-term effects of CPAP on pulmonary development. Future studies should investigate how CPAP could contribute to the pathophysiology of lung function in the presence of other clinically relevant scenarios including supplemental O2 and/or infection. Further work is needed to understand the mechanistic pathways contributing to the AW hyper-reactivity to determine potential longer-term consequences of neonatal CPAP in preterm infants with respiratory distress syndrome.
Methods
Subjects
Time-pregnant mice (C57BL/6J) were purchased from a commercial vendor (Charles River, Willmington, MA) and were later observed to give birth in our animal facility. Mice were treated with or without CPAP in 21% O2 for the first week of postnatal life; the lungs were removed in preparation for the measurement of AW reactivity using the living lung slice preparation the day after CPAP ended (P7) or 2 wk later (P21). All procedures were carried out in accordance with the US National Institute of Health (Bethesda, MD) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Neonatal CPAP Treatment
Following the day of birth (P0), the litter was divided in half and both male and female pups were randomly assigned to receive either CPAP or no CPAP starting the following day (P1) for the first 7 postnatal days. An example of how CPAP was delivered on the first postnatal day is provided in Figure 5 . The pups and the dam were maintained in a temperature controlled room during a 12:12 h, light:dark cycle and provided food and water ad lib. Each day the mice were removed from the dam and fitted with a custom made mask to fit snuggly around the head for administering CPAP ( Figure 5 ). CPAP was administered to unanesthetized mice, while placed on a temperature controlled heat pad (Gaymar T/pump, Orchard Park, NY) for temperature support. The mice appeared to tolerate the mask and CPAP well and settled down quickly after an initial few minutes of restlessness. The mask was designed with an entry port to deliver a flow of humidified air, which passed through to an exit port that was connected with tubing to a downstream manometer. An adjustable leak positioned in the downstream tubing between the mask and the manometer enabled continuous flow through the mask, while also allowing fine-adjustment of CPAP to be delivered directly to the mask (and the AWs of the mouse). Adjusting the leak to reduce the air flow through the mask provided a measureable back-pressure into the mask and to the manometer, which was used to continuously monitor the amount of CPAP for the duration of treatment. Although the mask created a snug fit around the face of the animal, it was loose enough that on some occasions, the mouse was able to maneuver free, after which it was immediately repositioned on the mask. CPAP was maintained at 6 cmH2O by adjusting the downstream leak or the upstream flow into the mask. CPAP lasted just 2 h for the first day to minimize the duration the pups were separated from the mother, but was increased to 3 h/session for the following 6 consecutive days (7 d total). An elaborate set up was designed to enable simultaneous delivery of CPAP to multiple mice (up to eight at a time). Control mice were also removed from the dam, fitted with the same masks, placed on the same heat pad, received the same airflow, but did not receive CPAP. After each bout of CPAP, the mask was removed and the pups were returned to the mother to resume normal rearing. The exposure to CPAP and the application of the mask for control mice was repeated daily for 7 consecutive days from P1 through P7 ( Figure 5 ). At the end of the 7 d of CPAP, the lungs of the mice were prepared for measurement of AW reactivity the following day or allowed an additional 2 wk of uninterrupted maternal care. AW reactivity was assessed using the in vitro living lung slice preparation, as described previously for mature rodents (36,37). An additional group of male and female mice were also used to assess the effects of a shorter duration (4 d) of CPAP between P1–4 on AW responses to methacholine. AW reactivity in these mice was assessed at P21, ~17 d after CPAP treatment ended, enabling age-match comparison to the mice that received 7 d of CPAP.
Schematic of the setup used to administer continuous positive airway pressure (CPAP) noninvasively to neonatal mice. A collar is fitted over the face through which air flows to a downstream manometer. An adjustable leak allows optimization of the back-pressure (i.e., CPAP) to be applied to the mask and airways (AWs) of the mouse. The level of CPAP can be accurately measured using the manometer (ΔP, pressure difference) and adjusted accordingly. The protocol for CPAP delivery (indicated above) consisted of 6 cmH2O, 3 h/day, for 7 consecutive days from postnatal age 1–7 d (P1–P7). Airway responses to methacholine challenge were assessed in the in vitro living lung slice preparation the day after (P8) and ~2 wk after CPAP treatment ended. Note, however, CPAP was administered for only 2 h on the first day (P1) instead of 3 h to minimize the duration the litter was separated from the dam.
Measurement of AW Reactivity
The day after (P8) or 2 wk after (P21) CPAP treatment ended, mice were sacrificed via anesthetic overdose (intraperitoneal injection of a ketamine/xylazine mix (100 mg/kg/10 mg/kg, respectively) to prepare the lungs for in vitro measurements of AW reactivity to methacholine. After death was confirmed, the mouse was placed supine for cannulation of the trachea using a syringe loaded with agarose. The cannula (PE tubing diameter: 0.5 mm (P8) or 0.58 mm (P21), Clay Adams, Sparks, MD) was inserted through a small ventral incision through the neck and into the anterior most part of the trachea. The cannula was advanced ~3 mm and held securely in place with suture. Liquefied (40 °C) agarose (Invitrogen, Carlsbad, CA) was gently injected to inflate the lungs (0.4 ml for P8 mice; 0.8 ml for P21 mice) and the mouse placed in the refrigerator for 30 min to allow the agarose to cool and gel. The entire lung was then removed, placed on a vibratome (VT1000, Leica Microsystems, Wetzler, Germany), sliced into 300-μm sections, and immersed in DMEM + Pen/Strep (Life Technologies, Carlsbad, CA) solution for overnight incubation (5% CO2; 37 °C) to melt the agarose. The following day, the lung slices were then rinsed in Hank’s Balanced Salt Solution (HBSS) and mounted in an in vitro recording chamber for live imaging of AW responses to methacholine challenge.
For live imaging of individual AWs, the slices were covered with a thin lightweight sheet of mesh and a coverslip, which were held in place with silicone grease (Molykote, Dow Corning, Midland, MI). The recording chamber containing the slice was mounted on a microscope (DMLFS, Leica Microsystems, Wetzler, Germany) and perfused (7 ml/min) continuously with HBSS at room temperature using a perfusion pump (MPII, Harvard Apparatus, Holliston, MA). The microscope mounted with a camera (Rolera Fast, QImaging, Surrey, Canada) was used to identify individual AWs under 5× magnification. After an initial 3-min period of baseline, the chamber was perfused with increasing doses of methacholine and changes in AW lumen area were recorded continuously. The extent of AW constriction in response to increasing doses of methacholine (0.25, 0.5, 1, 2, 4, and 8 µmol/l; Sigma Aldrich, St Louis, MO) was determined at the end of a 2-min period of exposure at each dose. Image analysis software (ImageJ software) (38) was used to determine the luminal area (in pixels) at each dose of methacholine to determine the extent of AW constriction. The greater the decrease in luminal area, the more reactive the AWs.
Individual AWs were chosen at random and the response to methacholine was performed on one AW/lung section, although we typically collected one to two sections per animal. Thus, treatment groups consisted typically of one to two AWs/animal spread across two to three litters. We categorized AWs into large or small size for each age group (i.e., P8 or P21) determined by averaging the largest and then the smallest AW lumen area to obtain a “middle” threshold. AWs with a baseline area above or below this value were then considered large or small, respectively (4,000 luminal pixel area for P8; 7,000 luminal pixel area for P21).
Radial Alveolar Counts
Radial alveolar counts were performed as previously described (10,38) on 21-d-old male mice. In brief, mice were euthanized with an intraperitoneal injection of ketamine/xylazine (Henry Schein, Melville, NY), the trachea was cannulated, and lungs were inflation-fixed (25 cm H2O) for 10 min with 10% neutral-buffered formalin (Electron Microscopy Sciences, Hatfield, PA). The left lung was removed, prepared for immunostaining, postfixed for 2 d at 4 °C and paraffin embedded. Sections were stained with hematoxylin and eosin (Fisher Scientific, Waltham, MA), and alveolarization was assessed by performing radial alveolar counts. All images were obtained using a Rolera XR CCD camera (Q-Imaging, Surrey, Canada) mounted on a Leica DMLB microscope (Leica Microsystems, Wetzler, Germany). Images were analyzed using research-based digital image analysis software (Image-pro Plus 7.0, Media Cybernetics, Silver Spring, MD). RAC was determined by counting the number of alveolar septa transected by a perpendicular line drawn from the terminal bronchiole to the nearest connective tissue septum (38), and was averaged from 8–10 sections per animal from each treatment group.
Data Analysis
Statistical comparison of responses to methacholine between control and CPAP treated groups was made using a two-way repeated measures ANOVA (SigmaPlot, Systat Software, San Jose, CA). Comparison of maximum contraction (at 8 µmol/l methacholine) between different size AWs or genders was performed using a one-way ANOVA. Statistical comparison of radial alveolar counts was performed between Ctrl and CPAP treated male mice using a t-test. Differences were considered significant at P < 0.05. All values for AW lumen area are expressed as mean ± SEM and (where relevant) presented in raw units (pixels) or as a fraction of initial baseline value.
Statement of Financial Support
This study was funded by the National Heart, Lung and Blood Institute (Bethesda, MD) Grant R01HL056470 (RJM) and the Department of Pediatrics, Rainbow Babies and Children’s Hospital, Cleveland, Ohio.
References
Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev 2009;85(10 Suppl):S1–3.
Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 2006;118:823–30.
Joshi S, Powell T, Watkins WJ, Drayton M, Williams EM, Kotecha S. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J Pediatr 2013;162:813–818.e1.
Sosenko IRS, Bancalari E. New developments in the pathogenesis, and prevention of bronchopulmonary dysplasia. In: Bancalari E, ed. The Newborn Lung: Neonatology Questions and Controversies. 2nd edn. WB Saunders: Philadelphia, 2010. pp. 187–207.
Laughon M, Allred EN, Bose C, et al.; ELGAN Study Investigators. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 2009;123:1124–31.
Belik J, Jankov RP, Pan J, Tanswell AK. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J Appl Physiol (1985) 2003;94:2303–12.
Denis D, Fayon MJ, Berger P, et al. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr Res 2001;50:515–9.
Hershenson MB, Abe MK, Kelleher MD, et al. Recovery of airway structure and function after hyperoxic exposure in immature rats. Am J Respir Crit Care Med 1994;149:1663–9.
O’Reilly M, Harding R, Sozo F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology 2014;105:39–45.
Wang H, Jafri A, Martin RJ, et al. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am J Physiol Lung Cell Mol Physiol 2014;307:L295–301.
Martin JG, Opazo-Saez A, Du T, Tepper R, Eidelman DH. In vivo airway reactivity: predictive value of morphological estimates of airway smooth muscle. Can J Physiol Pharmacol 1992;70:597–601.
Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998;275(1 Pt 1):L110–7.
O’Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 2008;177:1103–10.
Ramchandani R, Shen X, Elmsley CL, Ambrosius WT, Gunst SJ, Tepper RS. Differences in airway structure in immature and mature rabbits. J Appl Physiol (1985) 2000;89:1310–6.
Donn SM, Sinha SK. Minimising ventilator induced lung injury in preterm infants. Arch Dis Child Fetal Neonatal Ed 2006;91:F226–30.
Sinha SK, Donn SM. Newer forms of conventional ventilation for preterm newborns. Acta Paediatr 2008;97:1338–43.
Busk M, Busk N, Puntenney P, et al. Use of continuous positive airway pressure reduces airway reactivity in adults with asthma. Eur Respir J 2013;41:317–22.
Nandwani N, Caranza R, Hanning CD. Obstructive sleep apnoea and upper airway reactivity. J Sleep Res 1998;7:115–8.
Devouassoux G, Lévy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 2007;119:597–603.
Korczynski P, Gorska K, Przybylowski T, Bielicki P, Zielinski J, Chazan R. Continuous positive airway pressure treatment increases bronchial reactivity in obstructive sleep apnea patients. Respiration 2009;78:404–10.
Xue Z, Yu Y, Gao H, Gunst SJ, Tepper RS. Chronic continuous positive airway pressure (CPAP) reduces airway reactivity in vivo in an allergen-induced rabbit model of asthma. J Appl Physiol (1985) 2011;111:353–7.
Vrijlandt EJ, Gerritsen J, Boezen HM, Duiverman EJ ; Dutch POPS-19 Collaborative Study Group. Gender differences in respiratory symptoms in 19-year-old adults born preterm. Respir Res 2005;6:117.
McKenzie R, Burton MD, Royce SG, Tang ML. Age and sex influences on airway hyperresponsiveness. J Asthma 2010;47:651–4.
Diblasi RM, Zignego JC, Tang DM, et al. Noninvasive respiratory support of juvenile rabbits by high-amplitude bubble continuous positive airway pressure. Pediatr Res 2010;67:624–9.
Fukunaga T, Davies P, Zhang L, Hashida Y, Motoyama EK. Prolonged high intermittent positive-pressure ventilation induces airway remodeling and reactivity in young rats. Am J Physiol 1998;275(3 Pt 1):L567–73.
Polglase GR, Hillman NH, Ball MK, et al. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr Res 2009;65:67–71.
Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol (1985) 1996;81:1471–6.
Xue Z, Zhang W, Desai LP, Gao H, Gunst SJ, Tepper RS. Increased mechanical strain imposed on murine lungs during ventilation in vivo depresses airway responsiveness and activation of protein kinase Akt. J Appl Physiol (1985) 2013;114:1506–10.
Tsuchida S, Engelberts D, Roth M, McKerlie C, Post M, Kavanagh BP. Continuous positive airway pressure causes lung injury in a model of sepsis. Am J Physiol Lung Cell Mol Physiol 2005;289:L554–64.
Bäckström E, Hogmalm A, Lappalainen U, Bry K. Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr Res 2011;69:312–8.
Liu M, Skinner SJ, Xu J, Han RN, Tanswell AK, Post M. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol 1992;263(3 Pt 1):L376–83.
Laughon MM, Langer JC, Bose CL, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011;183:1715–22.
Regal JF, Lawrence BP, Johnson AC, Lojovich SJ, O’Reilly MA. Neonatal oxygen exposure alters airway hyper-responsiveness but not the response to allergen challenge in adult mice. Pediatr Allergy Immunol 2014;25:180–6.
Neriishi K, Frank L. Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am J Physiol 1984;247(3 Pt 2):R475–81.
Speyer CL, Rancilio NJ, McClintock SD, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 2005;288:C881–90.
Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 2002;119:187–98.
Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol 2002;283:L1271–9.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5.
Acknowledgements
We would like to thank Julie Di Fiore for a careful critique during preparation of this manuscript and Anjum Jafri for assistance with immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayer, C., Martin, R. & MacFarlane, P. Increased airway reactivity in a neonatal mouse model of continuous positive airway pressure. Pediatr Res 78, 145–151 (2015). https://doi.org/10.1038/pr.2015.90
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.90
This article is cited by
-
Prematurity-associated wheeze: current knowledge and opportunities for further investigation
Pediatric Research (2023)
-
Calcium-sensing receptor and CPAP-induced neonatal airway hyperreactivity in mice
Pediatric Research (2022)
-
CPAP-induced airway hyper-reactivity in mice is modulated by hyaluronan synthase-3
Pediatric Research (2022)
-
CPAP protects against hyperoxia-induced increase in airway reactivity in neonatal mice
Pediatric Research (2021)
-
Homöopathische Arzneimittel — Nachprüfungen
Archiv für Experimentelle Pathologie und Pharmakologie (1938)