Abstract
Background:
Heterozygous ATP-binding-cassette subfamily A member 3 (ABCA3) mutations are associated with neonatal respiratory complications. In an adult murine model, we investigated whether Abca3 haploinsufficiency is a predisposing factor for lung injury induced by hyperoxia or mechanical ventilation.
Methods:
Abca3 haploinsufficient (Abca3+/−) and wild-type (WT) mice were prospectively randomized to 25 min of ventilation or 72 h of hyperoxia or left unchallenged in air.
Results:
As compared with WT mice, unchallenged Abca3+/− mice had significantly decreased lung phosphatidylcholine (PC) and phosphatidylglycerol (PG) levels (P < 0.02) and decreased lung compliance (P < 0.05). When ventilated for 25 min, Abca3+/− mice demonstrated a significantly greater increase in bronchoalveolar lavage (BAL) interleukins (P ≤ 0.01) and lung wet to dry ratio (P < 0.005). Hyperoxia resulted in increased compliance (P < 0.05) and total lung capacity (TLC) (P = 0.01) only in the Abca3+/− mice, consistent with enlarged alveolar spaces. The ratio of PC to PG in BAL—relevant for surfactant dysfunction—was significantly elevated by oxygen exposure, with the greatest increase in Abca3+/− mice.
Conclusion:
In a murine model, Abca3 haploinsufficiency results in an altered biochemical and lung mechanical phenotype, as well as a greater lung injury induced by hyperoxia or mechanical ventilation. The inability to maintain a normal PC/PG ratio appears to play a key role.
Similar content being viewed by others
Main
The ATP-binding cassette subfamily A member 3 (ABCA3), a transport protein expressed mainly in the lamellar bodies of type II cells of the lung, plays an important role in surfactant metabolism and phospholipid homeostasis (1,2). Biallelic mutations that disrupt ABCA3 function are associated with fatal surfactant deficiency (3), whereas mild mutations may result in interstitial lung disease sometimes manifesting only during late childhood (4). Certain ABCA3 haplotypes are associated with respiratory distress in very premature infants (5). Defined heterozygous mutations also contribute to lung disease, the most common of them being E292V (4), found to be present in 4–7% of neonates with respiratory distress syndrome, with an incidence of 0.4–1.3% in the Caucasian population (6,7,8). One study estimated the frequency of a single mutation of ABCA3 to be 1.5–3.6% in African- and European-descent infants (representative of the general population) (9). The frequent occurrence of ABCA3 heterozygous mutations in the general population, although not causing disease in most, may be a predisposing factor for a more severe manifestation in a number of lung diseases.
We and others have previously reported that Abca3 knockout mice (Abca3−/−) do not survive the neonatal period due to absence of surfactant in the alveolar spaces, resulting in a failure of lungs to expand postnatally (10,11,12). In addition, a significant reduction of certain phospholipids such as phosphatidylglycerol (PG) and certain species of phosphatidylcholine (PC), which form a major part of the phospholipids found in pulmonary surfactant, is observed in the lung (11). Abca3 mice heterozygous for the null allele (Abca3+/−), although living and developing normally after birth, have variable development of lungs at birth and a slightly decreased number of lamellar bodies at 6 mo of age (12). In addition, in Abca3+/− mice, a reduced incorporation of phospholipid precursors into dipalmitoyl phosphatidyl choline, PC, and PG has been observed (12).
Previous experiments with mice haploinsufficient for type-II cell–specific proteins such as surfactant protein-B have shown a greater susceptibility to lung injury when exposed to factors such as hyperoxia (13). Exposure to either oxygen or mechanical ventilation causes changes in the lung, including increased inflammatory response, alveolar septal thickening, and breakdown of epithelial and endothelial barriers (14,15,16). Neonates with respiratory distress syndrome, as well as children manifesting with pulmonary disease, are frequently subjected to oxygen therapy and/or mechanical ventilation. Because up to 14.3% of such children might have a functionally significant single ABCA3 mutation (9), the elucidation of the effect of these therapies on those with heterozygous ABCA3 mutations is important. In this study, we evaluated the effect of hyperoxia for 72 h and/or mechanical ventilation for 25 min on mice haploinsufficient for Abca3. We hypothesized that exposure to hyperoxia or mechanical ventilation will cause greater injury to lungs of haploinsufficient mice as compared with wild-type (WT) mice.
Results
Lung Phospholipid Levels Are Altered in Abca3+/− Mice
Total lung PC 32:0 and PC 32:1, which are the major phospholipids in surfactant, were significantly decreased in Abca3+/− as compared with WT mice ( Figure 1 ). In addition, total lung PG was also significantly decreased in Abca3+/− mice (0.85 ± 0.16 vs. 0.98 ± 0.16; P = 0.01). Therefore, the lungs of Abca3+/− mice had a different phospholipid composition as compared with WT mice.
Major phosphatidylcholine species, namely PC 32:0 and PC 32:1, are decreased in lungs of Abca3+/− mice. Organic phospholipid extracts from mouse lungs, WT (white bars) and Abca3+/− (black bars) (n = 12 in each group), quantified by direct flow injection electrospray ionization tandem mass spectrometry for phosphatidylcholine, are expressed as nmol per mg wet weight of lung. The species of phosphatidylcholine are expressed on the x- axis as total acyl carbon:total double bond content. All values are mean ± SD. *P < 0.05; **P < 0.005. WT, wild-type.
Lung Mechanics of Abca3+/− Mice in Room Air Differ From Those of WT Mice
Abca3+/− mice maintained in room air had significantly lower dynamic compliance (C) as compared with WT mice ( Table 1 ). This translated into a significantly higher mean airway pressure in the Abca3+/− mice when ventilated in a volume-controlled manner with a tidal volume of 7 ml/kg. Abca3 haploinsufficiency status did not significantly affect other lung mechanics parameters such as total lung capacity (TLC), Newtonian resistance (Rn), or static compliance. Mechanical ventilation for 25 min decreased compliance in both and increased tissue elastance and tissue damping, but this was not significantly different in the two groups ( Table 1 ).
Mechanical Ventilation for 25 min Results in a Greater Inflammatory Response in Abca3+/− Mice as Compared With WT Mice
The effect of ventilation for 25 min, although resulting in increased bronchoalveolar lavage fluid (BALF) protein and interleukin levels in both groups of animals, was different in the WT and Abca3+/− mice ( Table 2 ). After ventilation for 25 min, lung wet to dry ratio as well as BALF interleukin (IL)-6 and C-X-C motif chemokine 2 precursor (CXCL-2) levels were significantly higher in the Abca3+/− mice as compared with WT mice ( Table 2 ). Histopathological examination of lungs exposed to 25 min of mechanical ventilation showed an increased median lung injury score of up to 3 for the Abca3+/− mice (n = 4) as compared with 0.5 in the WT animals (n = 4), although this did not reach significance.
Exposure to Hyperoxia
Exposure to 95% oxygen for 72 h was well tolerated by both Abca3+/− mice and WT mice, with no mortality in either of the groups. In the Abca3+/− mice, exposure to hyperoxia resulted in significantly higher lung wet to dry ratio as compared with WT mice, in which no change in the ratio was detected ( Table 3 ). Hyperoxia also resulted in increased BALF protein and interleukin levels in both groups. However, these changes were independent of the Abca3 genotype ( Table 3 ).
Lung Mechanics Measurements Demonstrated an Increased Total Lung Capacity and Compliance in Abca3+/− Mice Subjected to Hyperoxia
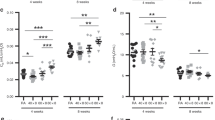
Abca3+/− mice had a significantly increased TLC as compared with WT mice after exposure to 72 h of hyperoxia ( Table 4 , Figure 2a – d ). When mice were ventilated for 25 min, this genotype-dependent difference persisted ( Figure 2b – d ). In addition, a significantly increased dynamic compliance was also observed in the Abca3+/− mice at 5 min of ventilation ( Table 4 ). Mechanical ventilation for 25 min after hyperoxia exposure resulted in increased BALF IL-6 and CXCL-2 in both WT and Abca3+/− mice, but these changes were not significantly different between the two groups (data not shown).
Changes in lung mechanics and histopathology after 72 h of hyperoxia. Lung mechanics measurement after 72 h of hyperoxia demonstrated an increased total lung capacity (TLC) only in the Abca3+/− mice (a: air, b: 95% oxygen, black line: Abca3+/− mice, dashed line: WT mice). (a,b) In WT mice, total lung capacity after hyperoxia was similar to that in air. All values are mean ± SD. *P = 0.01. Representative pressure–volume loops from each genotype also demonstrated an increased lung volume in (d) Abca3+/− mice as compared with (c) WT mice after hyperoxia (black line: pressure–volume loop at 5 min; gray line: pressure–volume loop at 25 min). Histopathological examination of the lung demonstrated an increased frequency of enlarged alveolar spaces in the (f) Abca3+/− mice as compared with (e) WT mice after hyperoxia (e and f, scale bar = 100 µmol/l). WT, wild-type.
Enlarged Alveolar Spaces Were Observed in the Lungs of Abca3+/− Mice After Hyperoxia
Because the changes in lung mechanics (increased TLC and lung compliance) could be due to emphysematous changes in the lungs, histopathology of the lungs was performed in mice with and without hyperoxia.
Lungs of Abca3+/− mice subjected to hyperoxia showed increased incidence of enlarged alveolar spaces as compared with those of WT mice ( Figure 2e , f ). The mean airspace chord length, representing the mean free distance in the air spaces, was also increased in the Abca3+/− mice, but this difference was not significant (WT = 57.0 ± 3.0, Abca3+/− = 63 ± 6.4, P = 0.14, n = 4 in both groups). The median lung injury score evaluated in the animals exposed to oxygen and ventilation was higher in the Abca3+/− mice as compared with WT mice (1.5 vs. 0; P = 0.04).
Hyperoxia Results in Significantly Reduced Amounts of Abca3 mRNA in Abca3+/− Mice
On investigating the effect of 72 h of 95% oxygen on Abca3+/− mice in terms of gene expression, it was observed that hyperoxia resulted in a decrease in Abca3 mRNA in both WT and Abca3+/− mice ( Figure 3a ). However, Abca3+/− mice exposed to hyperoxia demonstrated significantly lower levels of Abca3 mRNA as compared with WT-hyperoxia mice (P = 0.034) ( Figure 3a ). In addition, surfactant protein-B, surfactant protein-C, and surfactant protein-D mRNAs were significantly decreased in Abca3+/− mice exposed to hyperoxia as compared with Abca3+/− mice in air ( Figure 3b – d ). No significant change in these mRNAs was observed in WT mice.
Effect of hyperoxia on type II cell–specific mRNAs in wild-type (white box plots) and Abca3+/− mice (gray box plots). Seventy-two hours of hyperoxia resulted in a significant decrease in (a) Abca3, (b) surfactant protein-B, (c) surfactant protein-C, and (d) surfactant protein-D mRNA in the Abca3+/− mice. Abca3 mRNA was also decreased in the wild-type mice after hyperoxia, but the decrease in Abca3+/− mice was significantly greater. *P = 0.034, Abca3+/− mice as compared with wild-type mice. †P < 0.05, Abca3+/− mice in air vs. after hyperoxia.
BALF Phospholipid and Prosurfactant Protein B Levels Are Significantly Altered in Abca3+/− Mice
Abca3+/− mice maintained in air and ventilated for 25 min had significantly lower levels of certain subspecies of PG, such as 32:0, 32:1, 34:1, in BALF as compared with WT mice ( Figure 4b ). Hyperoxia resulted in significant changes in BALF PC and PG levels in WT and Abca3+/− mice ( Figure 4c , d ). Although in the WT mice, a significant increase in total polyunsaturated PC (constituted by PC 36:4, PC 38:6, PC 38:4, and so on) was observed with hyperoxia (10.0 ± 2.3 with hyperoxia vs. 5.4 ± 1.1 in air; P = 0.01), no increase was seen in the Abca3+/− mice (5.0 ± 0.4 with hyperoxia; P = 0.02 as compared with WT-hyperoxia). In addition, when exposed to hyperoxia, a significantly lower amount of all PG subspecies was observed in the Abca3+/− mice as compared with the WT mice ( Figure 4d ).
Bronchoalveolar lavage fluid (BALF) phosphatidylcholine (PC) and phosphatidylgylcerol (PG) levels in air and after exposure to hyperoxia. The difference in BALF PC and PG levels between wild-type mice (white bars) and Abca3+/− mice (black bars) was more pronounced after exposure to hyperoxia. (a,b) In air, (b) a significant difference between the geontypes was observed in some subspecies of PG, whereas (a) no significant difference was observed in the PC subgroups. However, (c,d) when exposed to hyperoxia, (c) all poly-unsaturated PCs (PC 36:4, 36:2, 38:6, 38:4) as well as PC 32:1, a major surfactant phospholipid was decreased in the Abca3+/− mice. (d) All subgroups of PG, the phospholipid most influenced by the level of Abca3 mRNA, were significantly lower in the BALF of Abca3+/− mice after hyperoxia exposure. All values are mean ± SE. *P < 0.05; **P < 0.01.
Increased BALF PC to PG ratio, relevant for surfactant dysfunction, was observed in WT mice when exposed to hyperoxia ( Table 5 ). In the Abca3+/− mice, however, a higher PC to PG ratio was observed even when they were maintained in air. Hyperoxia resulted in a further significant increase in PC/PG in the Abca3+/− mice ( Table 5 ).
Prosurfactant protein B (pro-SpB) expression was also significantly increased in BALF from Abca3+/− mice after hyperoxia exposure. The relative expression of pro-SpB was 15,270 ± 2,012 (mean ± 1 SD) arbitrary units in the WT mice as compared with 18,274 ± 1,500 in the Abca3+/− mice (n = 7 in each group; P = 0.02).
Discussion
In our study, we demonstrate that adult mice haploinsufficient for Abca3 are more susceptible to the deleterious effects of mechanical ventilation and hyperoxia as compared with their WT littermates. When subjected to mechanical ventilation, a significant increase in inflammatory response and wet-to-dry ratio of the lung is observed in the Abca3+/− mice as compared with WT mice. Hyperoxia for 72 h, on the other hand, results in enlarged alveolar spaces, with increased TLC and compliance on lung mechanic measurements and significant changes in BALF phospholipids, especially PG, only in the Abca3+/− mice.
Under normal physiological conditions, Abca3+/− mice have altered levels of PC 32:0, PC 32:1, and PG in the lung tissue ( Figure 1 ). In addition, a significantly altered PC to PG ratio is also observed ( Table 5 ). Dipalmitoyl phosphatidyl choline (PC 32:0) is considered to be the main phospholipid responsible for lowering the surface tension of alveoli to near-zero levels (17). An increased BALF PC to PG ratio, on the other hand, is associated with a lower amount of large surfactant aggregates in the BALF, translating into surfactant dysfunction and increased surface tension in the alveolar spaces (18).
In unchallenged Abca3+/− mice, although no significant difference in the Abca3 mRNA was observed between WT and haploinsufficient mice ( Figure 3a ), biochemically, a decrease in the levels of lung PC 32:0 and PC 32:1, and an altered BALF PC to PG ratio, was observed that translated into a significantly lower dynamic compliance with no other changes in lung mechanic measurements.
Mechanical ventilation for only 25 min resulted in significantly higher levels of IL-6, CXCL-2, and lung wet to dry ratio in Abca3+/− mice as compared with WT mice ( Table 3 ). It is possible that when Abca3+/− mice with decreased lung compliance are ventilated in a volume-controlled mode, it results in a greater lung injury due to the lungs being subjected to higher mean airway pressure required to deliver the same amount of tidal volume ( Table 2 ). Similar findings have been observed in cocultures of alveolar epithelial cells and fibroblasts, which, when subjected to higher pressure, resulted in a paracrine activation of fibroblasts and matrix proteins as well as ILs (19). The increased lung wet to dry ratio observed in the haploinsufficient mice is probably the result of damage to the fluid-reabsorptive sodium channels and/or Na-K-ATPase as a consequence of greater lung injury in accordance with increased inflammatory parameters (20). Therefore, Abca3+/− mice show a greater predisposition to lung injury when ventilated for only 25 min.
Seventy-two hours of hyperoxia in the Abca3+/− mice led to structural changes in the lungs as evident by increased TLC and enlarged alveolar spaces in the lungs. No such changes were observed in the WT mice, in which TLC after hyperoxia was not different from that in air ( Figure 2a , b , Tables 2 and 4 ). These findings in Abca3+/− mice are similar to those of the study by Besnard et al., in which mice with conditional knockout of Abca3 had decreased Abca3 and surfactant protein-B mRNA levels, and developed emphysema at the age of 9 mo in air (21). In Abca3+/− mice, however, exposure to hyperoxia for 72 h is sufficient to produce emphysematous changes in the lungs, which are also reflected by lung mechanics ( Figure 2 ). In rats exposed to hyperoxia, a decrease in lung collagen, as well as emphysematous changes in the lungs, was observed after 2 wk (22). This process is probably accelerated in the Abca3+/− mice, in which an initial surfactant dysfunction (increased PC to PG ratio in air) might result in a greater lung injury when exposed to hyperoxia, resulting in an even greater surfactant dysfunction ( Table 5 ).
Hyperoxia for 72 h decreased Abca3 mRNA in the lungs while producing no changes in the levels of the investigated surfactant protein mRNAs in the WT mice, which is similar to that seen in other studies with hyperoxia (23). However, Abca3+/− mice exposed to hyperoxia demonstrated a significantly greater decrease of Abca3 as compared with WT-hyperoxia mice. This decrease in Abca3 mRNA in the haploinsufficient mice with hyperoxia was associated with concomitant lower levels of all subspecies of PG ( Figure 4d ) and a significantly elevated PC to PG ratio almost twice that of WT in air ( Table 5 ). A significant decrease in lung PG levels has been demonstrated in Abca3 knockout mice (11). Therefore, we can expect haploinsuficiency of Abca3 to result in lower PG levels in the lung too. The difference in BALF PG levels was most prominent when the haploinsufficient mice were exposed to hyperoxia as compared with when they were maintained in air ( Figure 4b , d ).
A significant increase in pro-SpB levels was observed in the BALF of Abca3+/− mice with hyperoxia (P = 0.02). Elevation of pro-SpB levels has been seen after hyperoxia and may be associated with either a higher turnover or a greater sloughing off of cells due to hyperoxic injury (13). In full-term infants with fatal lung disease due to ABCA3 mutations, as well as in patients with pediatric interstitial lung disease with ABCA3 mutations, a similar increase in pro-SpB levels in the lung has been observed (4,24). In our model, the increase of pro-SpB in the BALF might be a combination of increased pro-SpB protein in the lungs (due to haploinsufficient genotype) as well as increased sloughing of cells due to increased tissue damage in Abca3+/− after hyperoxia.
Our study demonstrates for the first time that C57Bl/6 mice haploinsufficient for Abca3 are susceptible to hyperoxic and mechanical lung injury. Heterozygous ABCA3 mutations, especially E292V, are common in neonates with respiratory distress syndrome (6,7,9), and this is associated with a greater incidence of pneumothorax and chronic lung disease in very preterm neonates (6). In the newborn period, the pulmonary surfactant system is of particular relevance for the transition to air breathing. A reduced transport of PG into lamellar bodies, leading to reduced secretion into the BALF and thereby affecting PC to PG ratio and lung compliance, and increasing the predisposition to lung injury, appears to be the mode of action in haploinsufficient neonates. However, it is also possible that compensatory mechanisms induced as a result of the alteration of BALF PC to PG ratio might contribute to the increased susceptibility observed in haploinsufficient animals. Beyond the newborn period, ABCA3 haploinsufficiency may influence the clinical course of a number of lung diseases as well as acute or chronic lung injury as indicated by the findings of our study as well as by the observation in a clinical study that heterozygous ABCA3 mutations increase the severity of interstitial lung disease associated with surfactant protein C gene mutations (25).
Conclusion
Abca3 haploinsufficiency results in an alteration of phospholipid levels, BALF PC to PG ratio, and compliance in mouse lungs, and predisposes to lung injury by hyperoxia or mechanical ventilation.
Methods
Animal Care and Ethics Approval
All animal experiments were performed according to the Federal Act on the Protection of Animals (Germany) and approved by the committee of the District Government of Upper Bavaria (reference number: 55.2-1-54-2531-24-08). The animals were housed 4–5 per cage in a humidity- and temperature-controlled environment on a 12:12 h light–dark cycle, with free access to water and standard food.
Abca3 Haploinsufficient Mice
We have previously reported the generation of a C57BL/6-based mouse line carrying an Abca3-null allele (10). Haploinsufficient Abca3 mice (Abca3+/−) were maintained in conditions identical to WT mice.
Surfactant Phospholipid Analyses
Phospholipids were extracted from 2 mg of frozen lung tissue or 200 µl of BALF as previously described (26). Lipids were quantified by direct flow injection electrospray ionization tandem mass spectrometry in positive ion mode using the analytical setup and strategy as previously described (27,28). A precursor ion of m/z 184 specific for phosphocholine-containing lipids was used for phosphatidylcholine and sphingomyelin. Neutral loss scans were used for the following lipid classes: phosphatidylethanolamine 141, phosphatidylserine 185, PG 189, and phosphatidylinositol 277 (29,30).
Mechanical Ventilation Studies
Experiments were performed on Abca3+/− and WT mice at 10–12 wk of age. Mice from both genotypes were randomly assigned to either no mechanical ventilation (air) or mechanical ventilation for 25 min (air + ventilation), with the investigators blinded to the genotypes of the mice. In the “air + ventilation” group, a total of 22 WT mice and 13 Abca3+/− mice were enrolled, whereas in the “air” group, seven animals in each group were enrolled. The larger number of WT mice in the mechanical ventilation group was due to the fact that one batch of Abca3+/− mice could not be used for experiments due to technical reasons.
In the “air + ventilation” group, mice were anesthetized with a combination of medetomidine (0.5 mg/kg), midazolam (5 mg/kg), and fentanyl (0.05 µg/kg) and placed in a supine position. The mice were tracheotomized, cannulized with a 1.2-mm outer diameter steel cannula, and ventilated in a volume-controlled mode to achieve a tidal volume of 7 ml/kg and 180 breaths/min (SAV-Flexivent; SCIREQ, Montreal, Canada). Vecuronium bromide was used for muscle paralysis to ensure reproducible study conditions for lung mechanic measurements. Two lung recruitment maneuvers were performed before timed measurements in order to ensure comparable measurement of the lung. Data were recorded according to an automated protocol of lung mechanics measurement.
Lung Mechanics Measurement
During mechanical ventilation, automated lung function measurements were performed every 5 min using the flexiVent ventilator (SAV-Flexivent; SCIREQ). While measuring respiratory mechanics, the flexiVent briefly disrupted mechanical ventilation and executed four different automated measurement maneuvers (inspiratory plateau, P-V loop, snapshot, and forced oscillation), during which a predefined pressure or volume waveform was applied to the airway opening of the mice. TLC was measured by slowly inflating the lungs (3 s) until an inspiratory pressure of 30 cmH2O was reached. During the following hold at peak pressure (3 s), the lung volume reached an end-inspiratory plateau that reflected the TLC (31). Automated pressure-volume loops were used to capture the quasistatic mechanical properties of the respiratory system. These pressure-volume curves were not obtained by an occlusion technique originally described for the purpose of measuring static breath-by-breath compliance but by a stepwise inflation to TLC, measured at a lung pressure of 30 cmH2O, and deflation back to forced residual capacity. The quasistatic compliance (Cst) and elastance (Est) were then calculated using the software supplied with the ventilator from the slope of the Salazar–Knowles equation fitted to the deflation limb of the pressure-volume curve (32). Reproducible data for dynamic compliance and resistance were obtained by a sinusoidal waveform with precisely standardized amplitude and frequency. The frequency of this waveform was matched to the physiological respiratory rate of the mouse. Resistance (R) and compliance (C) were estimated on the basis of a single compartment model of the lungs as previously described (33). Rn, tissue damping (G), tissue elastance (H), and inertance (I) were obtained using the forced oscillation technique (33). Briefly, respiratory input impedance (Zrs) was measured between 0.5 and 20 Hz by applying a 16-s composite signal containing 19 mutually prime sinusoidal waves during pauses in regular ventilation. The peak-to-peak amplitude of the oscillatory signal was 50% of tidal volume. The constant-phase model described by Hantos et al. was used to partition Zrs into components representing the mechanical properties of the airways (Rn) and parenchyma (G, H) (34). Measurements were excluded if coherence was <90%.
Evaluation of Inflammatory Parameters in the Lungs
Both nonventilated animals and ventilated animals were killed with a lethal dose of pentobarbital before the thorax was opened, the lung mobilized, and the left main bronchus ligated. The right lung was lavaged with sterile phosphate-buffered saline. The samples were centrifuged, and the supernatant was used for measurement of protein by Bradford protein assay and interleukins (tumor necrosis factor-α; CXCL-2; IL-6) by multiplex-ELISA technology (Bio-PlexPro, Bio-Rad Laboratories, Hercules, CA). Total leukocytes were determined using a hemocytometer (Coulter, Krefeld, Germany) after resuspension of the cell pellets in 1 ml phosphate-buffered saline. Cellular populations were calculated in duplicates from differential cell counts in 400 µl fluid deposited onto glass slides using a cytospin centrifuge (1,000g for 10 min). The left lung was weighed directly after preparation, dried for 48 h at 65 °C, and weighed again to calculate the wet to dry ratio.
Exposure to Hyperoxia
Abca3+/− and WT C57BL/6 mice at 12–14 wk of age were placed with their cages in a Plexiglas incubator in which the oxygen concentration was maintained at 95% and 1 atmosphere for 72 h. Lung mechanics, lung wet to dry ratio, routine histological analyses, interleukin levels, and total protein content in BALF were determined as described above.
RNA Extraction and Real-Time PCR From Mouse Lung Tissue
RNA extraction with RNAzol followed the manufacturer’s protocol (WAK-Chemie Medical, Steinbach, Germany). RNA was extracted from the left lungs of mice kept in air or exposed to 95% oxygen (n = 4 in each group). Quantitative PCR was performed with 10 ng cDNA in a real-time PCR system (Step-one; Applied Biosystems, Life Technologies, Carlsbad, CA).
Primer sets used were as follows: Abca3 (exon 6, deleted in knockout allele), forward: CGAGGACTACATTCGCTATGAC, reverse: TGTAACTGAAGCGCAGGTG; surfactant protein B, forward: CACTGAGGATGCCATGGGCC, reverse: TGATCACAGACTTGCAGAAATGGCACT; surfactant protein C, forward: GGCCTTGCTGTGAGCACCCTG, reverse: CTTTCCTGTCCCGCTGCGGTT; surfactant protein D, forward: AGCGTCTAGAGGTTGCCTTCTCCC, reverse: TCCTGGGCATCCTCAAAAGGCT; Actin, forward: GTGGGCCGCTCTAGGCACCA, reverse: TGGCCTGAGGGTTCAGGG
Lung Histopathological Studies
In a subset of animals (from all groups), the right lung was expanded with 4% formalin and stored for at least 24 h in formalin solution. Following fixation, the lung lobe was embedded in paraffin, sectioned, and stained with hematoxylin–eosin for lung morphometry. Severity of lung injury was assessed using a scoring system that includes (i) alveolar and interstitial emphysema, (ii) tissue shrinkage, (iii) septal thickening, and (iv) infiltration or aggregation of polymorphonuclear cells. Each criterion was scored according to mild, moderate, or severe deviation. Five random fields of hematoxylin–eosin sections from each animal were examined in a blinded fashion, and a mean injury score between 0 and 12 was determined for each mouse. Representative lung sections with scores are shown in Supplementary Figure S1 online.
Morphometric Analysis of Lung Tissue
Design-based stereology was used to analyze hematoxylin and eosin-stained slides using an Olympus BX51 light microscope equipped with a computer-assisted stereological toolbox (CAST-Grid 2.1; Olympus, Ballerup, Denmark).
Western Blot Analysis
The content of pro-SpB was determined in the BALF by western blot analysis using standard procedures. Samples containing 1.25 pmol of saturated PC were loaded on the gel. Surfactant protein-B antibody, detecting the 42-kDa band representing pro-SpB, was used at a concentration of 1:1,000 (Acris Antibodies, Herford, Germany). Detection was achieved by appropriate goat anti IgG antibodies conjugated to horseradish peroxidase (1:7,500) using the SuperSignal West Femto detection system (Thermo Scientific, Rockford, IL). Chemiluminescence was monitored with a DIANA III imaging system (Raytest USA, Wilmington, NC), and bands were quantified using AIDA Image Analyzer software (Raytest USA, Wilmington, NC).
Statistical Analysis of Data
Statistical analyses were performed using commercially available statistics packages (Statgraphics, Statistical Graphics, Rockville, MD, and PAWS Statistics 18.0, IBM SPSS Software, IBM, Germany).
The data generated in the two groups after exposure to the different conditions (ventilation and/or oxygen treatment) were tested for normal distribution (Kolmogorov–Smirnoff) before a one-way ANOVA with Bonferroni post hoc testing was performed to compare the two groups. If terms of normal distribution were not met, the differences between the genotypes were evaluated with the Kruskal–Wallis test. P values <0.05 were considered statistically significant. Results are presented as mean and 1 SD.
Statement of Financial Support
The study was supported by grant HO 2519/2-1 from the German Research Council (Deutsche Forschungsgemeinschaft) awarded to A.H. and M.H.d.A. and by grant 01GS0850 from the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) through NGFN-Plus (National Genome Research Network) to M.H.d.A. and O.E.
Disclosure:
The authors declare that no financial ties or potential/perceived of conflicts of interest are present.
References
Yamano G, Funahashi H, Kawanami O, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett 2001;508:221–5.
Garmany TH, Moxley MA, White FV, et al. Surfactant composition and function in patients with ABCA3 mutations. Pediatr Res 2006;59:801–5.
Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M . ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med 2004;350:1296–303.
Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM . ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med 2005;172:1026–31.
Karjalainen MK, Haataja R, Hallman M . Haplotype analysis of ABCA3: association with respiratory distress in very premature infants. Ann Med 2008;40:56–65.
Garmany TH, Wambach JA, Heins HB, et al. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res 2008;63:645–9.
Hamvas A, Cole FS, Nogee LM . Genetic disorders of surfactant proteins. Neonatology 2007;91:311–7.
Bækvad-Hansen M, Nordestgaard BG, Dahl M . Heterozygosity for E292V in ABCA3, lung function and COPD in 64,000 individuals. Respir Res 2012;13:67.
Wambach JA, Wegner DJ, Depass K, et al. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics 2012;130:e1575–82.
Hammel M, Michel G, Hoefer C, et al. Targeted inactivation of the murine Abca3 gene leads to respiratory failure in newborns with defective lamellar bodies. Biochem Biophys Res Commun 2007;359:947–51.
Fitzgerald ML, Xavier R, Haley KJ, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res 2007;48:621–32.
Cheong N, Zhang H, Madesh M, et al. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem 2007;282:23811–7.
Tokieda K, Iwamoto HS, Bachurski C, et al. Surfactant protein-B-deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol 1999;21:463–72.
Smith LJ . Hyperoxic lung injury: biochemical, cellular, and morphologic characterization in the mouse. J Lab Clin Med 1985;106:269–78.
Crapo JD . Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 1986;48:721–31.
dos Santos CC, Slutsky AS . The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 2006;68:585–618.
Veldhuizen EJ, Haagsman HP . Role of pulmonary surfactant components in surface film formation and dynamics. Biochim Biophys Acta 2000;1467:255–70.
Hite RD, Seeds MC, Bowton DL, et al. Surfactant phospholipid changes after antigen challenge: a role for phosphatidylglycerol in dysfunction. Am J Physiol Lung Cell Mol Physiol 2005;288:L610–7.
Tschumperlin DJ, Drazen JM . Chronic effects of mechanical force on airways. Annu Rev Physiol 2006;68:563–83.
Zemans RL, Matthay MA . Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit Care 2004;8:469–77.
Besnard V, Matsuzaki Y, Clark J, et al. Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol 2010;298:L646–59.
Riley DJ, Kramer MJ, Kerr JS, Chae CU, Yu SY, Berg RA . Damage and repair of lung connective tissue in rats exposed to toxic levels of oxygen. Am Rev Respir Dis 1987;135:441–7.
Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M . C/EBP{alpha} is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol 2009;297:L286–98.
Brasch F, Schimanski S, Mühlfeld C, et al. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med 2006;174:571–80.
Bullard JE, Nogee LM . Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res 2007;62:176–9.
Mahavadi P, Korfei M, Henneke I, et al. Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. Am J Respir Crit Care Med 2010;182:207–19.
Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G . High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta 2004;1686:108–17.
Leidl K, Liebisch G, Richter D, Schmitz G . Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim Biophys Acta 2008;1781:655–64.
Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD . Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA 1997;94:2339–44.
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D . Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 2008;49:1137–46.
Flemmer AW, Jani JC, Bergmann F, et al. Lung tissue mechanics predict lung hypoplasia in a rabbit model for congenital diaphragmatic hernia. Pediatr Pulmonol 2007;42:505–12.
Lovgren AK, Jania LA, Hartney JM, et al. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2006;291:L144–56.
Bates JH . Understanding lung tissue mechanics in terms of mathematical models. Monaldi Arch Chest Dis 1993;48:134–9.
Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ . Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–78.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1.
(JPEG 160 kb)
Rights and permissions
About this article
Cite this article
Herber-Jonat, S., Mittal, R., Huppmann, M. et al. Abca3 haploinsufficiency is a risk factor for lung injury induced by hyperoxia or mechanical ventilation in a murine model. Pediatr Res 74, 384–392 (2013). https://doi.org/10.1038/pr.2013.127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.127