Abstract
Background:
Intrauterine growth restriction (IUGR) is known to affect the risk of adult diseases. Consumption of lipogenic fructose is increasing, and it is used as an enhancer of metabolic syndrome in rat experiments. The effects of IUGR, postnatal fructose diet, and their interaction on the lipid profile and adiposity were studied in adult rats.
Methods:
IUGR was induced by providing pregnant rats with 50% of daily food intake. From 1 mo onward, half of the offspring received a fructose-rich diet and were then followed to the age of 1 and 6 mo, when plasma lipid, glucose, and insulin levels were measured. The adipose tissue was visualized by magnetic resonance imaging at the age of 6 mo.
Results:
IUGR and fructose diet decreased body weight in adult rats. IUGR increased low-density lipoprotein cholesterol in 6-mo-old rats. The fructose diet evoked hypertriglyceridemia and hyperinsulinemia in both the sexes and decreased fasting glucose levels in female rats. Postnatal fructose diet increased lipid content percentage in the retroperitoneal and intra-abdominal adipose tissues in male rats. Interactions between IUGR and postnatal fructose diet were observed in adult weight in males.
Conclusion:
These results demonstrate the importance of IUGR and fructose diet in adverse changes in lipid and glucose metabolism.
Similar content being viewed by others
Main
The terms “programming,” “fetal origins hypothesis,” and “metabolic imprinting” are used to describe the conditions in the uterus that can lead to possibly permanent or at least long-term changes in the systems involved in growth and metabolism (1,2). Epidemiological studies indicate that low birth weight is related to the risk of metabolic syndrome, type 2 diabetes, and atherosclerosis in adulthood. Poor fetal growth has also been linked to adult obesity, hypertension, and abnormal lipid metabolism (3).
There is an interaction between the prenatal and postnatal environments on the health outcome of the offspring (3). Some, but not all, of the infants born small for gestational age show catch-up growth. Those experiencing early catch-up growth tend to have a higher BMI and fat mass as children (4), and according to some studies, they are at an increased risk for adult diseases as compared with those not showing catch-up growth (5). Therefore, it is interesting to elucidate the possible interactions between the prenatal growth and postnatal environment and their effects on the metabolic profile of the offspring.
Fructose is a highly lipogenic sugar, and its consumption increases plasma triglyceride and low-density lipoprotein (LDL) cholesterol levels in humans (6). In rats, prolonged feeding of fructose induces moderate hypertension, glucose intolerance, insulin resistance, hyperinsulinemia, and hypertriglyceridemia, all of which are signs of metabolic syndrome (7,8). Therefore, fructose-fed rats are often used as a model of the metabolic syndrome (9).
We performed a study of fetal growth restriction in rats. Initially, we aimed to determine if intrauterine growth restriction (IUGR) could affect plasma cholesterol and glucose levels and adiposity in adult rats. Then, we tested whether fetally growth-restricted rats as compared with rats with normal growth are more prone to deleterious effects of fructose. IUGR has been shown to influence the development of the islets of Langerhans in rats and therefore impair their capacity to secrete insulin (6). On the other hand, fructose consumption has been shown to cause insulin resistance in rats (5). Finally, the effects of long-term consumption of fructose on the metabolic syndrome phenotype were elucidated.
Results
Effects of IUGR
Growth of offspring. One-day-old fetally undernourished offspring were smaller than control offspring (males: mean weight of CC litters (dams fed ad libitum with their own pups), 6.61 (SD: 0.36) g and RR litters (food-restricted dams with their own pups), 5.76 (SD: 0.91) g; t-test; P < 0.001; females: CC litters, 6.22 (0.50) g and RR litters, 5.37 (0.90) g; t-test; P < 0.001). The proportion of liver and heart weight to total body weight was calculated, and no differences were observed between CC and RR groups (data not shown).
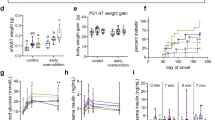
The weights of rat offspring from birth to 6 mo of age are presented in Figure 1 . In the age of 1 mo the weight of the IUGR pups did not differ from the weight of control pups. At the age of 6 mo, both male and female IUGR pups were smaller than control pups according to three-way ANOVA analysis ( Figure 2 , P < 0.001 and P < 0.001, respectively).
Growth of (a) male and (b) female rat offspring. Black squares, CC; black triangles, RR; black diamonds, RC. Postnatally, standard diet–fed rats are marked with solid lines and black symbols and fructose diet–fed rats with dashed lines and gray symbols. The symbols of rats that were fed with fructose diet were shifted right to clarify the figure. Individual weight data were available from 1-mo-old rats (n = 10–14 rats per study group) and 6-mo-old rats (n = 12–14 rats per study group), and they were analyzed by ANOVA. Results are represented as mean ± SE. Data from time points 2–5 mo are mean ± SE from six to seven litters per study group and were not statistically analyzed because of the lower dispersion due to loss of individual weight data. CC, rat offspring from dams fed ad libitum; RC, fetally undernourished rats raised by dams fed ad libitum during pregnancy; RR, fetally undernourished rats raised by their own mothers. *P < 0.001 for fetal undernutrition effect, **P < 0.05 for suckling period effect, †P = 0.012 for fructose diet effect, and ‡P = 0.020 for interaction between IUGR and fructose diet.
Weight of the rat offspring at the age of 6 mo. (a) Weight of male rats and (b) weight of female rats. White boxes represent rats that had been postnatally maintained on standard diet and gray boxes represent rats maintained on the fructose diet. The black squares indicate means and the whiskers indicate the range from minimum to maximum values. In male and female offspring, P < 0.001 for the effect of IUGR and P = 0.027 and P = 0.045 for the effect of suckling period, respectively. In male offspring, P = 0.012 for the effect of the fructose diet and P = 0.020 for the interaction between IUGR and the fructose diet. IUGR, intrauterine growth restriction.
Lipid and glucose metabolism. The effects of fetal undernutrition on the plasma lipid levels of 1- and 6-mo-old offspring were examined. At the age of 1 mo, total cholesterol levels were higher in IUGR female rats (CC: 2.64 (0.36), RR: 2.94 (0.30), and RC (dams fed ad libitum with pups born from food-restricted dams): 2.99 (0.59) mmol/l; P = 0.013). The increase in total cholesterol was also seen in fetally undernourished male rats, but the difference was not statistically significant (CC: 2.77 (0.29), RR: 3.00 (0.27), and RC: 2.86 (0.29) mmol/l; P = 0.305). Moreover, the difference in high-density lipoprotein (HDL) cholesterol in IUGR female rats reached statistical significance (CC: 0.90 (0.24), RR: 1.04 (0.26), and RC: 1.20 (0.69) mmol/l; P = 0.045).
The effects of fetal undernutrition on plasma lipid levels in the 6-mo-old male and female rats are shown in Tables 1 and 2 . Fetally undernourished rats had significantly higher LDL cholesterol levels (males: P < 0.001 and females: P = 0.001) and tended to have a higher total cholesterol level than control rats. In 1-mo-old rats, there were no differences in fasting glucose and insulin levels between the study groups (data not shown). Fasting glucose and insulin levels of 6-mo-old rats are shown in Tables 1 and 2 . Due to IUGR, male offspring were more insulin sensitive on the basis of the homeostasis model of assessment of insulin resistance (P = 0.041).
Magnetic resonance imaging results. Magnetic resonance imaging (MRI) for 6-mo-old CC and RR rats was performed. The MRI sequence for fat/water detection was based on selective excitation of the fat and water nuclear magnetic resonances. Lipid content percentages of retroperitoneal, intra-abdominal, and subcutaneous adipose tissues according to MRI are shown in Table 3 . There was no difference in the lipid content percentages of the tissues between the CC and RR groups.
Effects of the Suckling Period
Growth of offspring. The suckling period reflects the time after birth until weaning. Lactating dams had been either food-restricted during pregnancy (RR group) or normally fed (CC and RC groups). The suckling period had significant effect on the weight of the female offspring at 1 mo of age and on the weight of both the sexes at 6 mo of age ( Figure 1 ; P < 0.001 and Figure 2 ; P = 0.027 and P = 0.045, respectively). Offspring that were lactated by food-restricted dams were smaller as compared with other offspring.
Lipid and glucose metabolism. The suckling period had significant, increasing effect on plasma triglyceride levels in 6-mo-old male RR offspring ( Table 1 ; P = 0.019). There was no effect on triglyceride levels in female rats, but LDL cholesterol was increased in female offspring lactated by food-restricted dams ( Table 2 ; P = 0.004). Glucose metabolism was not affected by the suckling period.
Effects of Fructose Diet
Growth of offspring. The fructose diet did not induce weight gain in control or fetally undernourished rats ( Figure 1 ). The fructose-fed, 6-mo-old male rats were smaller than the rats that received standard diet ( Figure 2 ; P = 0.012).
Lipid and glucose metabolism. The effects of postnatal fructose diet on plasma lipid levels of 6-mo-old male and female rats are shown in Tables 1 and 2 . The fructose diet increased plasma total cholesterol, triglyceride, and HDL cholesterol levels in female rats (P = 0.004, P < 0.001, and P = 0.001, respectively). It increased triglyceride levels and decreased LDL levels in male rats (P < 0.001 and P < 0.001, respectively).
Fasting glucose was lower in female rats that had consumed the fructose-rich diet ( Table 2 ; P = 0.008) but not in male rats ( Table 1 ; P = 0.841). Fasting insulin was higher in male and female rats on the fructose diet (P = 0.012 and P = 0.030, respectively). Fructose impaired insulin sensitivity in 6-mo-old male rats (P = 0.026) but did not have any effect on insulin sensitivity in female rats (P = 0.382).
MRI results. The lipid content percentages of retroperitoneal, intra-abdominal, and subcutaneous adipose tissues are shown in Table 3 . The postnatal fructose diet increased the amount of lipids in retroperitoneal and intra-abdominal adipose tissues in male rats (P = 0.008 and P = 0.024, respectively). There were no differences in lipid content percentages in female rats.
Interactions Among IUGR, Suckling Period, and Fructose Diet
There was an interaction effect between IUGR and fructose diet in adult weight ( Figure 2 ; P = 0.020) in male rats, with the CC group fed with standard diet being the heaviest of all groups. Moreover, in male and female offspring, there was an interaction effect between suckling period and fructose diet in LDL cholesterol level ( Tables 1 and 2 ; P = 0.004 and P = 0.013, respectively). The RR group with standard diet had the tendency to highest LDL cholesterol levels, whereas the RR group consuming the fructose diet had the lowest LDL cholesterol levels.
Discussion
The aim of the study was to determine the effects of IUGR and postnatal fructose diet on plasma lipid levels and glucose and fat accumulation in rat offspring. Therefore, we followed the fetally undernourished rats to the age of 6 m. In addition, we determined if undernourished rats would be more prone to postnatal fructose-induced changes in adulthood. Earlier studies have shown that impaired fetal growth can increase the risk of metabolic syndrome in later life. Fructose consumption has also been shown to induce metabolic syndrome.
We were interested to determine whether fetally undernourished pups would have accelerated growth after birth and display the so-called catch-up growth. Previously, rapid catch-up growth has not been found in all IUGR studies (10) but is often seen in studies in which offspring are nursed by dams fed ad libitum (11,12). In our study, the fetally undernourished rats were smaller than controls at birth, although male rats did not differ in weight at the age of 1 mo. However, the fetally undernourished rats did not gain more weight from 1 to 6 mo as compared with the control rats. At 6 mo, the fetally undernourished rats were smaller than control rats. We could not observe catch-up growth, whereas we found that fetal undernourishment resulted in decreased weight both in female and male rats at the age of 6 mo.
Caloric restriction in the intrauterine period seemed to affect the lipid metabolism in growing (1 mo old) and adult (6 mo old) rats. Plasma LDL cholesterol levels at the age of 6 mo in both sexes were mostly affected by IUGR. In humans, similar results have been obtained (13,14). In previously reported studies on rats, there are differences between the experimental designs, and the results are difficult to compare. Often, the triglycerides are increased in IUGR animals with rapid catch-up growth (11). However, in our study, the catch-up growth and increase in triglycerides in IUGR rats were not seen. Instead, we found that male RR offspring, which were fetally undernourished and lactated by their own dams, had higher plasma triglyceride levels than the CC and RC offspring, highlighting the effect of suckling dam. IUGR could affect lipid metabolism possibly through altered liver growth or liver development or altered endogenous cholesterol synthesis or absorption. In addition, epigenetic mechanisms may be a part of the programming, given that, e.g., a recent report revealed histone modifications surrounding the Cyp7a1 promoter in hypercholesterolemic IUGR pups of protein-restricted dams (15). We did not determine how the food restriction during pregnancy affected the milk quality of the lactating dams. It may have an effect, given that the RR offspring were still smaller than the other offspring at the age of 6 mo. However, the study design is somewhat imperfect at this point because a study group in which offspring of control mothers were lactated by food-restricted mothers was not included, and this may bias the result from the suckling effect result.
The method by which IUGR is induced may confer some variability to studies. In most of the animal studies related to IUGR, the maternal nutritional availability has been manipulated to create offspring that are small at birth. Maternal caloric and protein restriction are the most widely used methods. In some studies, the growth of rat fetuses has been restricted by unilateral or bilateral uterine artery ligation in late gestation, which has led to impaired glucose tolerance in adult life (16). In addition, the effects of postnatal growth patterns vary between artery ligation studies, and there are also differences between the studies in the nutrition during lactation. Evidence for catch-up growth after lactation has (17,18) or has not been obtained (16). However, the majority of these studies have reported impaired glucose tolerance in male progeny by the time they reach adulthood (16,17,18).
There is also variability in the timing of nutrient restrictions during the pregnancies. In the studies of Desai et al. (11,12), the nutrient restriction started from day 10 of pregnancy when the placenta is fully developed, whereas in our study, food restriction affected the development of the placenta because the diet restriction started from day 4 of pregnancy. It can be hypothesized that the development of the placenta was somehow adjusted when the nutrient supply was restricted. In that way, it might be that the fetus does not need to adapt in such a drastic manner. The rats born in our study were, however, smaller than control rats.
The effect of IUGR on glucose and insulin metabolism depends on the timing of IUGR during pregnancy. When IUGR was induced during the first 2 wk of pregnancy in an earlier study, insulin secretion and insulin action were not changed in male offspring (19). In the recent study of Lim et al. (20), the Wistar Kyoto rats with low-protein diet during the whole pregnancy gave birth to offspring that had persistently increased whole-body insulin sensitivity and lower body weight in the absence of postnatal catch-up growth in adulthood. In our study, male IUGR offspring showed increased insulin sensitivity. Reduced β-cell mass and insulin content in adult offspring are observed when nutrient restriction occurs during the last trimester of pregnancy (19). However, opposite results also exist. For example, in the study of Bertin et al. (21), glucose tolerance, utilization, or production was not impaired in female offspring by IUGR induced in the last trimester of pregnancy.
Overall, the severity of the impact of IUGR to the offspring has varied extensively between the reported studies. The reason for this is unknown, but it can be hypothesized that the genetic variability among Sprague Dawley colonies might also affect the results.
The fructose diet had major effects on the plasma lipid profile reflected as an increase of triglycerides and LDL cholesterol in male rats and total cholesterol, triglycerides, and HDL cholesterol in female rats after consuming the fructose-rich diet for 5 mo. An effect of fructose feeding on plasma triglycerides has also been reported in many studies on rats (7,22). Increased lipogenesis in the liver and reduced triglyceride clearance may explain the results (6). The high levels of free fatty acids produced in de novo lipogenesis may alter glucose metabolism and contribute to hyperinsulinemia and insulin resistance (23). This hyperinsulinemia and insulin resistance was evident in our male rats fed with the fructose diet, whereas females displayed only higher insulin levels. Fructose also decreased fasting glucose levels in female rats, which is an unexpected finding and needs further studies. Higher plasma HDL cholesterol level in female offspring due to fructose feeding is an interesting but unexpected finding.
Our study suggests that fructose consumption may specifically promote lipid deposition in retroperitoneal and intra-abdominal adipose tissues. This is interesting because the consumption of fructose in sweeteners and drinks is increasing (24) and the increased visceral fat is seen as a risk for the metabolic syndrome. Our study indicates that high, long-term consumption of fructose does not induce weight gain, whereas it increases the amount of lipids in visceral, metabolically most deleterious adipose tissue in the body, making the effects of a fructose diet especially harmful. In one study, there was a decrease in peroxisome proliferator-activated receptor-α activity, and therefore, decreased fatty acid oxidation and increased lipid accumulation was induced by fructose feeding (25), which might be one mechanism to explain our results.
There are differences in the amounts of fructose used in chow between studies conducted, which complicates any comparison of the data. In many studies, fructose has induced weight gain, such as in the study of Meirelles et al. (26), in which the rats of the groups consuming 20% fructose solutions exhibited weight gain (27). However, there are studies with opposite results in which the body weight did not change (28,29) or decreased (30). In the recent study of Shapiro et al. (31), the feeding of fructose did not result in gaining body weight, although in that study, fructose induced leptin resistance, which could favor the positive energy balance. In our study, fructose increased the amount of lipids in retroperitoneal and intra-abdominal adipose tissues only in males. In addition, the rise in plasma triglycerides and insulin resistance after fructose consumption was much stronger in males as compared with females, which might be due to protective effects of estrogen. Earlier studies have indicated that estrogen can exert protective effects against insulin insensitivity provoked by high-fructose feeding (9).
In none of the previous studies have the possible interactions of the maternal caloric restriction, suckling period, and later postnatal fructose feeding been studied. There is possibly an interaction between IUGR and postnatal fructose feeding. IUGR offspring were smaller than the offspring from CC mothers. However, fructose seems to attenuate the weight difference induced by IUGR. The latter phenomenon is caused possibly by the slower weight gain in all fructose-fed animals. For LDL cholesterol, there seems to be an interaction between suckling period and fructose diet. Moreover, earlier studies suggest the role of lactation in the adult energy metabolism and response to different diets (32). The RR group with standard diet had the tendency to highest LDL cholesterol levels, whereas the RR group consuming the fructose diet had the lowest LDL cholesterol levels. This could be due to the production of large, triglyceride-rich very-low-density lipoprotein particles not converted to LDL but cleared from plasma by the remnant receptors. One study elucidated the interaction between maternal protein restriction and immediate postnatal fructose, and it was concluded that the offspring were not more susceptible to the effects of fructose diet (33).
The number of rats examined in our study is relatively high as compared with the previous studies. In this study, the inclusion of both male and female rats is also an advantage. However, the limitations of our study are the absence of the quantitation of whole-body fat and determination of food intake given that IUGR and early postnatal period may have affected appetite. In addition, not all the groups were studied by MRI. When analyzing the effect of suckling period, it would have been statistically better to establish a study group in which offspring from control dams were lactated by dams that were food-restricted during pregnancy.
In conclusion, impaired fetal growth leads to metabolic abnormalities seen mainly as changes in plasma cholesterol. Fructose feeding seems to cause hypertriglyceridemia and hyperinsulinemia. Having both impaired fetal growth and a fructose diet does not seem to be more harmful than having only one of these conditions. In summary, this study provides information on the consequences of fetal growth deprivation with or without long-term postnatal fructose consumption to adult health in rats.
Methods
Experimental Design and Diets
Sprague Dawley rats were obtained from the Laboratory Animal Centre of the University of Oulu. The virgin rats were mated at the age of 9 wk. Schematic representation of the study design is shown in Figure 3 . From day 4 of gestation, a group of dams received standard laboratory chow (Harlan Teklad Global 18% Protein Rodent Diet—3.1 kcal/g energy density, 24% of calories from protein, 18% from fat, and 58% from carbohydrates; Harlan Teklad, Indianapolis, IN) ad libitum, whereas the other group received only 50% of the ad libitum food intake until delivery. Normal daily food intake was determined previously (34). Rats were housed separately with free access to water during 12/12 h light/dark cycles. After delivery, all dams and offspring received standard chow ad libitum. On postnatal day 1, the litter sizes were equalized to eight pups (four males and four females) per litter, and the following study groups were assigned: dams fed ad libitum with their own pups (CC), dams fed ad libitum with pups born from food-restricted dams (RC), and food-restricted dams with their own pups (RR). The offspring were weighed three times a week and weaned on postnatal day 23.
At the age of 1 mo, seven CC litters, five RC litters, and six RR litters were studied as follows: Two male and two female pups from each litter were anesthetized by isoflurane inhalation, and blood was obtained by cardiac puncture after which the rats were killed by decapitation during the light phase between 12 AM and 2 PM The pups had fasted for 4 h. Blood was collected into heparinized vacutainers and centrifuged. Plasma was removed and stored at −70 °C for later use.
From 1 mo of age onward, half of the litters of each study group received a fructose-rich diet (Harlan Teklad TD89247 60% Fructose Diet— 3.6 kcal/g energy density, 20.2% of calories from protein, 12.9% from fat, and 66.8% from carbohydrates; Harlan Teklad), and the other half continued receiving the standard diet. At the age of 6 mo, the same procedures as described above were conducted and samples were taken as previously except that the rats were fasted for 12 h. The experimental design was approved by the Animal Experiment Board in Finland.
Laboratory Analyses
Plasma concentrations of total cholesterol and triglyceride levels were measured by an enzymatic colorimetric method (Roche Diagnostic, Mannheim, Germany). In the determination of HDL cholesterol concentration, 0.5 ml of plasma was mixed with 2.5 μl of 2.8% (wt/vol) heparin and 5 μmol manganese chloride, incubated at 4 °C for 30 min and centrifuged at 1,000g at 4 °C for 30 min. The HDL cholesterol concentration was measured from the supernatant at a wavelength of 490 nm. LDL was calculated using the Friedewald formula (35). Blood glucose was measured by Contourmeter (Bayer HealthCare, Mishawaka, IN). Blood was collected into heparinized vacutainers, centrifuged, and supernatant was removed to receive plasma. Plasma samples were stored at −70 °C for later use. Plasma insulin was measured by commercial enzyme-linked immunosorbent assay kit (Merck Millipore, Billerica, MA). Insulin resistance was assessed with the homeostasis model assessment of insulin resistance index, calculated as insulin (ng/ml) × glucose (mmol/l)/22.5.
MRI
MRI was performed for 6-mo-old rats (n = 4 males and females per group from the CC and RR study groups). MRI was conducted using a 4.7 T Magnex horizontal magnet (Magnex Scientific, Abington, UK) interfaced to a Varian UNITYINOVA console (Varian, Palo Alto, CA) with a transmit/receive quadrature volume RF coil (Rapid Biomedical, Rimpar, Germany). The MRI sequence for fat/water detection was based on selective excitation of the fat and water nuclear magnetic resonances (36). The imaging sequence was a three-dimensional gradient echo (repetition time: 100 ms, echo time: 12 ms, and total imaging time: 28 min) and for each point in the k-space, fat and water signals were selectively excited and acquired. The excitation for fat was set at −680 Hz (3.4 ppm) from water. The field-of-view was 60 × 60 × 100 mm3, and the matrix size was 256 × 128 × 128.
The lipid content was analyzed from the three-dimensional images by finding the net volumes of fat and water voxels, scaling these by the densities of fat and water and calculating the fat weight percentage. To exclude noise voxels, a threshold value was calculated for each slice: kthresh = 2 × Snoise + Smean, in which Smean is the average signal of the slice. Only the voxels above the threshold were included in the analysis. The shift in fat images (due to chemical shift difference from water) was corrected before the calculations. The range of axial images used for the analyses was manually selected from the image set covering the torso from sternum to distal pelvis. Regions of interest were manually drawn to retroperitoneal, intra-abdominal, and subcutaneous areas of adipose tissues. All data analyses were performed using the home-built image processing software Aedes (http://aedes.uku.fi) and another in-house MATLAB application (MathWorks, Natick, MA).
Statistical Analysis
Three-way ANOVA was used when the effects of fetal undernutrition (IUGR or normal conditions during pregnancy), suckling period (lactating dam food-restricted during pregnancy or not), postnatal diet (fructose-rich or standard chow from 1 mo to 6 mo of age), and their interactions were studied between the study groups. Two-way ANOVA was used only when the effect of IUGR and suckling period on the body weight of 1-mo-old rats was studied and when analyzing results obtained from MRI. Logarithmic transformation was applied to normalize distributions when needed. A P value smaller than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software package version 15.0 (SPSS, Chicago, IL).
Statement of Financial Support
The study was supported by Finnish Foundation for Cardiovascular Research.
Disclosure
The authors declared no conflict of interest.
References
Lucas A . Programming by early nutrition: an experimental approach. J Nutr 1998;128:Suppl 2:401S–6S.
Barker DJ . The origins of the developmental origins theory. J Intern Med 2007;261:412–7.
McMillen IC, Robinson JS . Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005;85:571–633.
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB . Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 2000;320:967–71.
Leunissen RW, Stijnen T, Hokken-Koelega AC . Influence of birth size on body composition in early adulthood: the programming factors for growth and metabolism (PROGRAM)-study. Clin Endocrinol (Oxf) 2009;70:245–51.
Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K . Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E685–94.
Hwang IS, Ho H, Hoffman BB, Reaven GM . Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987;10:512–6.
Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW . Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr 1989;49:1155–63.
Tran LT, Yuen VG, McNeill JH . The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 2009;332:145–59.
Woodall SM, Johnston BM, Breier BH, Gluckman PD . Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res 1996;40:438–43.
Desai M, Gayle D, Babu J, Ross MG . The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol 2007;196:555.e1–7.
Desai M, Gayle D, Babu J, Ross MG . Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 2005;288:R91–6.
Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH . Growth in utero and serum cholesterol concentrations in adult life. BMJ 1993;307:1524–7.
Martyn CN, Gale CR, Jespersen S, Sherriff SB . Impaired fetal growth and atherosclerosis of carotid and peripheral arteries. Lancet 1998;352:173–8.
Sohi G, Marchand K, Revesz A, Arany E, Hardy DB . Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol 2011;25:785–98.
Siebel AL, Mibus A, De Blasio MJ, et al. Improved lactational nutrition and postnatal growth ameliorates impairment of glucose tolerance by uteroplacental insufficiency in male rat offspring. Endocrinology 2008;149:3067–76.
Simmons RA, Templeton LJ, Gertz SJ . Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001;50:2279–86.
Nüsken KD, Dötsch J, Rauh M, Rascher W, Schneider H . Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 2008;149:1056–63.
Portha B, Chavey A, Movassat J . Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res 2011;2011:105076.
Lim K, Armitage JA, Stefanidis A, Oldfield BJ, Black MJ . IUGR in the absence of postnatal “catch-up” growth leads to improved whole body insulin sensitivity in rat offspring. Pediatr Res 2011;70:339–44.
Bertin E, Gangnerau MN, Bailbé D, Portha B . Glucose metabolism and beta-cell mass in adult offspring of rats protein and/or energy restricted during the last week of pregnancy. Am J Physiol 1999;277(1 Pt 1):E11–7.
Maiztegui B, Borelli MI, Raschia MA, Del Zotto H, Gagliardino JJ . Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol 2009;200:139–49.
Jürgens H, Haass W, Castañeda TR, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res 2005;13:1146–56.
Tappy L, Lê KA . Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 2010;90:23–46.
Roglans N, Vilà L, Farré M, et al. Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology 2007;45:778–88.
Meirelles CJ, Oliveira LA, Jordão AA, Navarro AM . Metabolic effects of the ingestion of different fructose sources in rats. Exp Clin Endocrinol Diabetes 2011;119:218–20.
Abdulla MH, Sattar MA, Johns EJ, Abdullah NA, Hye Khan MA, Rathore HA . High-fructose feeding impacts on the adrenergic control of renal haemodynamics in the rat. Br J Nutr 2012;107:218–28.
Lee YC, Ko YH, Hsu YP, Ho LT . Plasma leptin response to oral glucose tolerance and fasting/re-feeding tests in rats with fructose-induced metabolic derangements. Life Sci 2006;78:1155–62.
Faure P, Rossini E, Lafond JL, Richard MJ, Favier A, Halimi S . Vitamin E improves the free radical defense system potential and insulin sensitivity of rats fed high fructose diets. J Nutr 1997;127:103–7.
Lin S, Yang Z, Liu H, Tang L, Cai Z . Beyond glucose: metabolic shifts in responses to the effects of the oral glucose tolerance test and the high-fructose diet in rats. Mol Biosyst 2011;7:1537–48.
Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ . Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 2008;295:R1370–5.
Palou M, Torrens JM, Priego T, Sánchez J, Palou A, Picó C . Moderate caloric restriction in lactating rats programs their offspring for a better response to HF diet feeding in a sex-dependent manner. J Nutr Biochem 2011;22:574–84.
Cambri LT, Ghezzi AC, Ribeiro C, Dalia RA, de Mello MA . Recovery of rat growth and lipid profiles in adult rats subjected to fetal protein malnutrition with a fructose-rich diet. Nutr Res 2010;30:156–62.
Hietaniemi M, Malo E, Jokela M, Santaniemi M, Ukkola O, Kesäniemi YA . The effect of energy restriction during pregnancy on obesity-related peptide hormones in rat offspring. Peptides 2009;30:705–9.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
Thomas EL, Saeed N, Hajnal JV, et al. Magnetic resonance imaging of total body fat. J Appl Physiol 1998;85:1778–85.
Acknowledgements
The authors thank Saija Kortetjärvi for expert laboratory assistance and Johanna Närväinen, Biomedical Imaging Unit, University of Eastern Finland, for magnetic resonance imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malo, E., Saukko, M., Santaniemi, M. et al. Plasma lipid levels and body weight altered by intrauterine growth restriction and postnatal fructose diet in adult rats. Pediatr Res 73, 155–162 (2013). https://doi.org/10.1038/pr.2012.173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.173
This article is cited by
-
Fetal growth restriction followed by early catch-up growth impairs pancreatic islet morphology in male rats
Scientific Reports (2023)
-
Mitochondrial 2,4-dienoyl-CoA reductase (Decr) deficiency and impairment of thermogenesis in mouse brown adipose tissue
Scientific Reports (2019)
-
Prenatal caloric restriction alters lipid metabolism but not hepatic Fasn gene expression and methylation profiles in rats
BMC Genetics (2017)
-
Maternal dietary free or bound fructose diversely influence developmental programming of lipogenesis
Lipids in Health and Disease (2017)
-
Fatty Acid de Novo Synthesis in Adult Intrauterine Growth‐Restricted Offspring, and Adult Male Response to a High Fat Diet
Lipids (2016)