Abstract
We questioned to what extent changes in temperature could affect the newborn's ventilatory inhibition provoked by lung inflation, or Hering-Breüer (HB) inflation reflex. Conscious newborn rats (3-4 d old) were studied in a double chamber airflow plethysmograph at ambient temperatures of 32°C (slightly below their thermoneutrality), 24°C (cold), and 36°C (warm). At these ambient temperatures, the corresponding body temperatures averaged 35.4, 31.0, and 37°C. The HB reflex was triggered by negative body surface pressures of 5 or 10 cm H2O, and quantified as the duration of the expiratory time during the maintained inflation, either in absolute values or in relation to the control expiratory time. In cold the HB reflex decreased to 80%, and in warm it increased to 150%, of the values measured at 32°C. Oxygen consumption, measured by an open flow system, averaged 59, 47, and 29 mL·kg-1·min-1 at, respectively, 24, 32, and 36°C. In cold, the ventilatory response to hypoxia (10% O2) was almost absent, that to hypercapnia (5% CO2) was greater, and that to hypoxia and hypercapnia combined was less than in warm. We conclude that in newborn rats the strength of the vagal inhibition on breathing, evaluated in the form of the HB reflex, is sensitive to temperature, becoming stronger as temperature increases. One contributing factor is the temperature-induced change in metabolic rate, whereas the role of temperature-induced changes in ventilatory chemosensitivity is variable. The strong temperature-dependency of the neonatal HB reflex implies that in newborns exposed to a warm environment breathing is more susceptible to inhibitory inputs.
Similar content being viewed by others
Main
The work of Hering and Breüer, 130 years ago, has indicated the importance of the vagal pulmonary feedback in modulating the breathing pattern. Airways stretch receptors are activated in inspiration by the progressive increase in lung volume and transpulmonary pressure, and their inputs eventually stop further inspiratory activity, beginning the expiratory phase. When lung volume is maintained on an elevated state, the continuous firing of the stretch receptors delays the onset of the next inspiration (HB expiratory promoting reflex)(1). The strength of the reflex depends on the interplay of numerous factors. In addition to the degree of stimulation of the airways receptors, the chemical drive that gradually builds during the ventilatory inhibition is important in offsetting the vagal inputs(2). Because the chemical drive results from the tissues' V˙O2 and V˙CO2, the metabolic level is also expected to play role. Hence, depression of metabolism or chemosensitivity, for example, by anesthesia, increases the strength of vagal inhibition(3,4). On the other hand, in hypoxic conditions the increased chemical drive reduces the strength of the HB reflex(3).
In newborn rats, the effects of hypoxia on the vagal inhibition were found to depend on postnatal age; at 8 d after birth, hypoxia and hypercapnia decreased the strength of the HB reflex, as in adults, but this was not the case at younger ages(5). Differences in chemosensitivity, and their interaction with the hypoxia-induced changes in metabolic rate, were considered an important aspect of these developmental differences. In fact, the response to chemical stimuli is almost absent in the very first few days after birth and increases rapidly in the early postnatal phase(5,6).
In newborns, hypoxia readily decreases metabolic rate, and this, depending on the duration of the hypometabolism and the heat capacitance, results in a drop of Tb(7). Furthermore, several observations suggest that in newborns, as in adults, hypoxia lowers the set point of thermoregulation, such that a normal thermal environment during normoxia can be perceived as a hyperthermic condition during hypoxia(8–10). Hence, a better understanding of the ventilatory inhibition of pulmonary origin in the newborn requires some knowledge of the effects of changes in temperature on these reflex responses. In the present study we measured the HB reflex in conscious normoxic newborn rats at 32, 36, and 24°C Ta, examining the hypothesis that metabolic rate contributes to the strength of the reflex. We then attempted to interpret the results in light of the effects of changes in Ta on metabolic rate, Tb, and ventilatory chemosensitivity to hypoxia and hypercapnia, singly or combined.
METHODS
Experiments were performed on newborn Sprague-Dawley rats, at 3 and 4 d of age (day of birth = d 0), either in the morning or afternoon, after approval from the Animal Ethics Committee of McGill University. The pregnant dams had free access to food and water, and were maintained in individual cages at Ta between 22 and 25°C, 50-53% relative humidity, 12 h/12 h dark-light cycle.
The main experimental protocol consisted of measurements of the HB reflex for a period of 20 min at the Ta of 32°C, which is slightly below thermoneutrality for rats of this age(11); in half the rats measurements were then repeated during a 20-min exposure to 24°C (cold), and in the other half during a 20-min exposure to 36°C (warm). Additional groups of rats were used measurements of Tb, gaseous metabolism, and ventilatory chemosensitivity, at the same Ta and following the same time sequence of the main protocol.
Effects of changes of Ta on Tb. The animals were placed in the same setup used for the measurements of the HB reflex (see below), with the chambers preset at 32°C by a large-size heating lamp. Rectal temperature was measured with a very fine tungsten-constantan thermocouple (DP30, Omega, Stamford, CT) inserted about 15 mm in the rectum, and its value was taken as representative of Tb. After 20 min the chamber Ta was increased to either 36°C (warm) or lowered to 24°C (cold) by, respectively, adjusting the distance of the heating source or cooling the animal chamber with ice-cold wet pads. Values of Ta and Tb were registered every 2 min, for the entire 40-min experiment. After switching from Ta of 32°C to warm or cold, Ta reached the desired new value within 5 min.
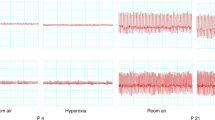
Hering-Breüer reflex. The setup (Fig. 1, top)consisted of a double-chamber plethysmograph similar to the one previously described(5). The animal was placed in the back chamber with its head emerging into the front chamber through multiple layers of paraffin sealing film (Parafilm). The two chambers were therefore separated from each other. The front chamber (approximately 15 mL) was for measurements of the breathing pattern, via a small pneumotachograph connected to a differential pressure transducer (Validyne, Northridge, CA) for recording of the V˙ signal, which was electronically integrated to obtain Vt. A steady flow of 80 mL/min, controlled by a flowmeter, was continuously delivered through the chamber, to avoid any accumulation of expired air.
(Top) Schematic representation of the experimental apparatus. (Bottom) Records of P, V˙, and VT in two rat pups with different breathing patterns (left: 4-d-old, 7.5 g, at 36°C; right: 3-d-old, 11 g, at 32°C). As P is lowered, lung volume is increased, triggering an inhibition of inspiratory activity that lasts until overcome by the chemical drive (HB reflex).
The back chamber (approximately 30 mL volume) was for the application of negative P (i.e. positive transrespiratory system pressures). To this end, the chamber had two openings; one was normally open to the ambient air, but could be rapidly switched to a vacuum source by turning a stopcock. The second opening was connected to a polyethylene tube the resistance of which was adjusted to obtain the desired chamber pressure (equivalent to P) during application of the vacuum. P was continuously monitored by a Validyne pressure transducer. The three signals (V˙, Vt, P) were recorded on paper (Gould pen recorder) at a speed of 25 mm/s. Both the front and back chambers had a small tungsten-constantan thermocouple for measurements of Ta. The pup was placed in the setup with the chambers preset at 32°C. After 5 min, P of 5 or 10 cm H2O were applied in random order for the next 15 min, at approximately 1-min intervals, during quiet breathing. Ta was then changed to either 36°C (warm, n = 15) or 24°C (cold, n = 15). After 5 min, the inflation maneuvers were again repeated for the next 15 min. The total experiment lasted 40 min, at which time Tb was measured and the pup returned to the dam.
As P was applied, lung volume increased, and breathing temporarily stopped until the chemical drive was strong enough to overcome the vagal inhibition. When gross body movements occurred during the inflation maneuver they were readily recognizable, and these runs were discarded. The HB reflex was quantified (Fig. 1, bottom) as 1) the total time from beginning of the expiration immediately before the application of P to the onset of the next inspiration during the maintained inflation (Teinfl), and 2) as "inhibitory ratio," i.e. the ratio between Teinfl and the average expiratory time (Te) of the five breaths preceding the inflation(5). This analysis was performed by use of a graphics tablet connected to a minicomputer.
In a separate group of four animals, the reflex was studied with Ta maintained constant at 32°C for 60 min, and the results for the first 20 min were compared with the last 20 min. This was done to test the possibility of a time-dependent change in the reflex during a 60-min period.
Gaseous metabolism. V˙O2 and V˙CO2 were measured with an open flow system, with a setup and a methodology similar to that previously adopted(11). Briefly, rat pups in sets of four were placed in the metabolic chamber preset at Ta of 32°C, through which a constant V˙ of 235 mL/min was continuously delivered. The outflow gas circulated through a drying column and was sampled by appropriate gas analyzers for measuring O2 and CO2 concentrations; the corresponding inflow concentrations were checked intermittently. Values were displayed on a computer monitor, and V˙O2 and V˙CO2 were computed during several minutes as the product between the inflow-outflow gas concentration difference and the flow. After 20 min, Ta was changed to either the warm or cold condition, and gaseous metabolism measured again during the next 20 min. Metabolic data are presented normalized by the weight in kilograms, at standard temperature and pressure, dry (STPD) conditions. At the end, Tb was measured in each pup.
Ventilatory response to hypoxia and hypercapnia. The rat was placed in an airflow plethysmograph, with the head emerging through several layers of Parafilm(12,13). The general setup was similar to that described above for the HB measurements, except that in this case V˙ and Vt were monitored from a pneumotachograph connected to the back chamber, whereas the front chamber was for the continuous delivery of air (15 min) followed by an additional 10 min of either hypercapnia (5% CO2 in normoxia), hypoxia (10% O2 balanced with N2), or moderate asphyxia (10% O2 and 5% CO2). With the animal quiet, toward the end of each exposure, approximately 100-150 continuous breaths were recorded on paper at a speed of 10 mm/s. Finally, Tb was measured and the pup returned to the dam. For each rat, ventilatory responses were measured twice, in cold and warm conditions, at d 3 or 4, in random order. These records were analyzed with a graphics tablet connected to a minicomputer for computation of the average Vt, instantaneous f, and V˙E (VT × f). Volume data (VT and V˙E) were normalized by the weight of the animal in kilograms.
Number of animals and statistical analysis. The total number of pups studied was 133, from 27 litters. Number of animals, or sets of animals in the case of metabolic measurements, used for each experiment is given in Table 1, and in the pertinent sections of "Results." Data are presented as group means ± 1 SEM. The statistical significance of differences between two groups of data (e.g. in warm and cold) was assessed by two-tailed t test, either paired or unpaired, as appropriate. A significant difference between three sets of data (warm, 32°C, and cold) was assessed by ANOVA followed by post hoc test with Bonferroni's limitations for the three comparisons. In all cases, a significant difference was considered at p < 0.05.
RESULTS
Effects of changes of Ta on Tb. At Ta of 32°C, Tb averaged 35.4 ± 0.2°C. By the end of the warm and cold phases Tb averaged, respectively, 37.1 ± 0.3°C and 31.0 ± 0.6°C (Fig. 2, "basal measurements," open circles). In warm and cold, at the end of the various tests (measurements of metabolic rate, HB reflex, and ventilatory chemosensitivity), Tb values were in general close to these basal measurements (Fig. 2, filled symbols). However, Tb values after the ventilatory responses to hypoxia and hypercapnia in cold tended to be lower, by about 2°C, probably because the cold exposure during these tests lasted slightly longer than in all the others (25 min instead of 20 min).
Average Tb of rat pups at 3-4 d of age, at Ta of 32°C (n = 9), 36°C (warm, n = 5), and 24°C (cold, n = 5). For both cold and warm, mean values of Tb immediately after the end of the measurements of metabolic rate (n = 44), HB reflex (n = 15), and hypoxic (n = 15), hypercapnic (n = 15), and asphyxic ventilatory responses (n = 15) are also indicated. Oblique line is the identity line. Bars indicate 1 SEM. * significant difference from 32°C, ‡ significant difference from cold.
Hering-Breüer reflex as function of time. The strength of the HB reflex measured during the first 20 min did not differ significantly from that measured in the last 20 min of a 1-h experiment. For example, at P of 5 cm H2O, the inhibitory ratios of the first and last 20 min averaged, respectively, 5.7 ± 0.5 and 5.7 ± 0.7.
Hering Breüer reflex as function of Ta. The inhibition of inspiratory activity during maintained lung inflation, whether measured as absolute time (Fig. 3, bottom) or as inhibitory ratio (Fig. 3, top), was greater at higher (10 cm H2O) than at lower (5 cm H2O) inflating pressures. At either pressure, changes in Ta resulted in important changes in the strength of the HB reflex (Fig. 3). In comparison with the value at 32°C, in warm the inhibitory ratio increased by about 50% (p < 0.005, average of the two distending pressures), whereas in cold it decreased by about 20% (p < 0.005). On average, HB reflex evaluated as Teinfl at 36°C was 231% of the 24°C value. Similarly, the HB reflex evaluated as inhibitory ratio at 36°C was 188% of the 24°C value (p < 0.001).
Effects of changes in Ta (32, 24, and 36°C) on the HB reflex. The reflex was evaluated as the Te (bottom) or as the inhibitory ratio (top) at inflation pressures of 5 and 10 cm H2O. A total of 30 rats were studied at 32°C; of these, 15 were studied also at 24°C, and the remaining 15 at 36°C. Symbols represent group means, bars indicate 1 SEM. * significant difference from 32°C, ‡ significant difference from 24°C.
Gaseous metabolism. As expected(11) metabolic rate was higher the lower the Ta (Fig. 4). At 32°C, V˙O2 averaged 47 ± 2 mL · kg-1 · min-1. In cold it increased to 59 ± 2 mL · kg-1 · min-1, whereas in warm it dropped to 36 ± 2 mL · kg-1 · min-1. Hence, in cold, gaseous metabolism (V˙O2 and V˙CO2) was about 160% of the warm value (p < 0.001).
Ventilatory response to hypercapnia. Both during warm and cold, hypercapnia elicited an increase in V˙E, almost exclusively because of the rise in VT. The increase, however, was significantly larger at 24°C than at 36°C, whether expressed in absolute terms (712 and 380 mL · kg-1 · min-1 in cold and warm, respectively, p < 0.001) or as a percentage of the corresponding ventilatory values during air breathing (respectively, 157% and 140%, p < 0.005) (Fig. 5, top).
Average values of V˙E and VT during breathing air and breathing an hypercapnic (5% CO2, top), hypoxic (10% O2, middle), or asphyxic (5% CO2 and 10% O2, bottom) mixture, in warm (36°C, open symbols) or cold (24°C, filled symbols). Oblique lines are isofrequency lines at the f indicated. Symbols are group means of 15 rats each, bars indicate 1 SEM.
Ventilatory response to hypoxia. In warm, hypoxia increased V˙E to 159% of normoxia, because of an increase in both VT and f. In cold, on the other hand, neither V˙E, nor VT or f. changed significantly from normoxia (Fig. 5, middle).
Ventilatory response to asphyxia. In warm, asphyxia increased V˙E to approximately 200% of the resting value by significantly increasing both VT and f. In cold, V˙E increased to 169%, with no changes in f. Hence, the V˙E increase in warm was significantly greater than in cold (Fig. 5, bottom). The values V˙E achieved were very similar (2441 ± 57 and 2453 ± 132 mL · kg-1 · min-1 in cold and warm, respectively), raising the possibility that the lower percentage response in cold was constrained by the maximal achievable V˙E. Hence, in eight of these rats in warm we measured V˙E during a more severe asphyxia (6.5% CO2 and 7.2 O2%); in this condition, V˙E averaged 2855 ± 101 mL · kg-1 · min-1, which was significantly higher than during moderate asphyxia.
DISCUSSION
During lung inflation the stimulation of the airways stretch receptors causes a progressively greater inhibition of breathing, which culminates in the termination of inspiration and the onset of expiration. The prolonged expiratory phase when lung volume is maintained increased (HB reflex) is a manifestation of this reflex control of the breathing pattern, mediated by the pulmonary vagal afferents. The prolongation of expiration is counteracted by the inspiratory chemical drive i.e. the progressive decrease in PaO2 and rise in PaCO2(2,3). Both depend on metabolic rate (V˙O2 and V˙CO2), and their effectiveness in offsetting the vagal inhibition depends on the degree of chemosensitivity. Hence, temperature can potentially affect the HB reflex at various levels of the reflex loop and of its interaction with the chemical control. In the present study on conscious newborn rats we found that the net effect was an increase in the strength of the HB reflex with temperature. Some of the mechanisms for this phenomenon are clear, others remain speculative. Before discussing their contributions and implications, some aspects of the methodology and protocol here adopted will be briefly addressed.
Methodological considerations. Because newborns need frequent maternal care, tests had to be planned for rather short durations, and repeated at various times. This could have introduced variability, as metabolic rate, a key factor in the chemical control of breathing(14), changes with age and time of the day. We tried to minimize these potential problems by maintaining the age range as narrow as possible (2 d), and randomizing the morning-afternoon sessions for the various experimental tests. The HB reflex did not vary significantly during a 1-h period, which was longer than any of the tests performed. Because different tests had to be performed on different groups of animals, interindividual variability could have obscured some relationships. However, this was not a concern as none of the dependent variables had large variability.
The strength of the HB reflex was evaluated at fixed distending pressures, rather than at the same inflation volumes, to take into account the fact that the pulmonary stretch receptors are stimulated by changes in airways tension; in so doing, we have eliminated interpretative problems in the eventuality of changes in respiratory compliance(15).
Temperature and metabolic rate. An inverse relationship between V˙O2 and Ta is the general characteristic of the response to cooling in homeotherms, reflecting their thermogenic effort for the maintenance of Tb. Conversely, warming stimuli reduce thermogenesis and increase heat loss. Very young and small mammals are no exception. However, despite the thermoregulatory efforts, their Tb varies with Ta by a magnitude that depends on the intensity and duration of the thermal stimuli, the body heat capacitance, and heat dissipation mechanisms(11). Among the physiologic consequences of a change in Tb are those on metabolic rate itself, via the Q10 effect. Hence, a low Tb limits the thermogenic effort, whereas high Tb enhances V˙O2 even when thermogenesis is suppressed. In the present experiments with cooling to 24°C or warming to 36°C for 20 min, Tb varied from 31 to 37°C, and V˙O2 varied, respectively, from 59 (cold) to 36 (warm) mL · kg-1 · min-1, i.e. by about 60%. Comparable changes in metabolic rate must have occurred during the measurements of the HB reflex, because the duration of the exposures and the final Tb values were very similar.
Temperature, V˙E/V˙O2, and chemosensitivity. The notion that changes in Ta or Tb affect ventilatory chemosensitivity is supported by numerous experiments in anesthetized and conscious preparations, although the former may be of more complex interpretation because of the depressant effects of anesthesia on thermoregulation(7). In awake men hyperthermia increased the V˙E responses to hypoxia or hypercapnia(16,17). Similar information emerged from studies on conscious rats, which also showed depressed V˙E responses to hypoxia or hypercapnia in the cold(18). The reduced V˙E response to hypoxia in the cold can be largely attributed to the important decrease in metabolic rate(7), whereas the depressant effect of cold on the V˙E response to hypercapnia(19–22) occurs even in the absence of changes in metabolic rate. The present results indicating the absence of a hyperpneic V˙E response to hypoxia in cold rat pups are therefore in line with previous data, particularly in small newborns(14).
The effect of temperature on the response to hypercapnia, on the other hand, contradicts the adult data, because we found that the increase in V˙E whether in absolute values or as a percentage of the air-breathing value, was greater in cold than in warm Ta. From the average data of V˙E and V˙E2, it can be calculated that during cold, V˙E/V˙O2 was substantially less than in warm (respectively, 21 and 29), whereas in adults thermogenesis is usually characterized by parallel increases in metabolic rate and V˙E(7). Also on a previous occasion V˙E/V˙O2 of 6-d-old rats was found to decrease as Ta dropped from 33°C to 25°C(21). A likely reason for the decreased V˙E/V˙O2 in the cold is the important drop in Tb, about 6°C. In fact, a decrease in core temperature has been postulated to have a negative effect on V˙E(23). In conscious adult rats during moderate asphyxia, V˙E/V˙O2 in the cold was less than in warm conditions when their Tb was allowed to drop (by about 6°C), whereas it was the same when Tb was maintained at the warm level by an abdominal heat exchanger(22). In addition to the drop in Tb, the pups' age could also be a factor in determining the magnitude of the hypercapnic V˙E response in warm and cold. It is known that, in warm conditions, the rat's ventilatory chemosensitivity changes very rapidly during the first postnatal days(5,6), but whether these developmental changes are exactly the same when in combination with the cold is not known.
Factors contributing to the Ta sensitivity of the HB reflex. The Ta-induced changes in metabolic rate very likely contributed to the changes in the strength of the HB reflex. In fact, the drop in metabolism in the warm implied that the rise in PaCO2, and the drop in PaO2, during the apnea of the HB reflex occurred at lower rates, therefore delaying the apnea breaking point. On the other hand, the role of the asphyxic ventilatory drive during the HB reflex is more difficult to assess. In the present experiments, the Ta sensitivity to hypoxia and hypercapnia combined was higher in warm than in cold, and therefore should have limited the Ta-induced changes in HB reflex. However, because the Ta sensitivity of the V˙E responses to hypoxia and hypercapnia, considered separately, was qualitatively different, it is probable that the role of the asphyxia in determining the strength of the HB reflex may vary, not only quantitatively, but also qualitatively, with temperature.
In addition to the role played by metabolic rate and chemosensitivity, temperature may have affected the HB reflex by modifying the central effectiveness of the vagal inhibition both during inspiration and expiration. In adult anesthetized animals an increase in Tb increased the reflex expiratory prolongation(24), and the HB volume threshold terminating inspiration decreased, indicating a greater central effectiveness of vagal inhibitory inputs during hyperthermia(25–27).
Both vagal and carotid body afferents could change sensitivity with changes in temperature. Results obtained in adult animal preparations have indicated that decreases and increases in temperature have parallel effects on the activity of the airways stretch receptors(28,29), which should imply corresponding effects on the HB reflex. The activity of the afferents from the carotid bodies is less at lower temperatures(30,31). However, this may not be relevant to the early neonatal period, because at this time carotid body afferents are almost silent(32). Finally, because the intensity of the HB reflex can be weaker during REM sleep, or some phases of REM sleep, in comparison with non-REM sleep(33,34), one cannot exclude that changes in sleep organization may have contributed to the Ta effects on the HB reflex.
Conclusions. Despite the difficulties in sorting out the relative contributions of the various mechanisms and the possibility of their change with age, the present experiments in 3- to 4-d-old rat pups have indicated a strong temperature dependency of the HB reflexes inhibiting ventilation remains to be seen. Nevertheless, in view of the reports of high body or environmental temperature in some victims of sudden infant death(35,36), the suggestion that inputs inhibitory on breathing can become more powerful in hyperthermic conditions seems important within the clinical context of neonatal breathing irregularities and apneas.
Abbreviations
- f :
-
breathing frequency
- HB :
-
Hering-Breüer
- P :
-
body surface pressure
- Ta :
-
ambient temperature
- Tb :
-
body temperature
- TE :
-
expiratory time
- TE infl :
-
expiratory time during lung inflation
- V˙ :
-
airflow
- V˙E :
-
minute ventilation
- VT :
-
tidal volume
- V˙CO 2 :
-
gaseous carbon dioxide production
- V˙O 2 :
-
gaseous oxygen consumption
- REM :
-
rapid eye movement
References
Widdicombe JG 1964 Respiratory reflexes. In: Fenn WO, Rahn H (eds) Handbook of Physiology. Respiration. Am Physiol Soc, Washington DC, sect 3, vol 1 585–630.
Younes M, Vaillancourt P, Milic-Emili J 1974 Interaction between chemical factors and duration of apnea following lung inflation. J Appl Physiol 36: 190–201.
Bouverot P, Crance JP, Dejours P 1970 Factors influencing the intensity of the Breuer-Hering inspiratory-inhibiting reflex. Respir Physiol 8: 376–384.
Richardson PS, Widdicombe JG 1969 The role of the vagus nerves in the ventilatory response to hypercapnia and hypoxia in anaesthetized and unanesthetized rabbits. Respir Physiol 7: 122–135.
Matsuoka T, Mortola JP 1995 Effects of hypoxia and hypercapnia on the Hering-Breuer reflex of the conscious newborn rat. J Appl Physiol 78: 5–11.
Eden GJ, Hanson MA 1987 Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol 392: 1–9.
Mortola JP, Gautier H 1995 Interaction between metabolism and ventilation: effects of respiratory gases and temperature. In: Dempsey JA, Pack AI (eds) Regulation of Breathing. Marcel Dekker, New York, 1011–1064.
Pedraz C, Mortola JP 1991 CO2 production, body temperature and ventilation in hypoxic newborn cats and dogs before and after body warming. Pediatr Res 30: 165–169.
Rohlicek CV, Saiki C, Matsuoka T, Mortola JP 1996 Cardiovascular and respiratory consequences of body warming during hypoxia in conscious newborn cats. Pediatr Res 40: 1–5.
Mortola JP, Feher C 1998 Hypoxia inhibits cold-induced hudding in rat pups. Respir Physiol 113: 213–222.
Mortola JP, Dotta A 1992 Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am J Physiol 263:R267–R272.
Saetta M, Mortola JP 1985 Breathing pattern and CO2 response in newborn rats before and during anesthesia. J Appl Physiol 58: 1988–1996.
Saetta M, Mortola JP 1987 Interaction of hypoxia and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol 62: 506–512.
Mortola JP 1996 Ventilatory responses to hypoxia in mammals. In: Haddad GG, Lister G (eds) Tissue Oxygen Deprivation. From Molecular to Integrated Function. Marcel Dekker, New York, 433–477.
Gaultier C, Mortola JP 1981 Hering-Breuer inflation reflex in young and adult mammals. Can J Physiol Pharmacol 59: 1017–1021.
Petersen ES, Vejby-Christensen H 1977 Effects of body temperature on ventilatory response to hypoxia and breathing pattern in man. J Appl Physiol 42: 492–500.
Vejby-Christensen H, Petersen ES 1973 Effect of body temperature and hypoxia on the ventilatory CO2 response in man. Respir Physiol 19: 322–332.
Maskrey M 1990 Body temperature effects on hypoxic and hypercapnic responses in awake rats. Am J Physiol 259:R492–R498.
Ruiz AV 1975 Carbon dioxide response curves during hypothermia. Pflugers Arch 358: 125–133.
Gautier H, Bonora M, Trinh HC 1993 Ventilatory and metabolic responses to cold and CO2 in intact and carotid body-denervated awake rats. J Appl Physiol 75: 2570–2579.
Saiki C, Mortola JP 1996 Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol 491: 261–269.
Mortola JP, Maskrey M 1998 Ventilatory response to asphyxia in conscious rats: effect of ambient and body temperatures. Respir Physiol 111: 223–246.
Gautier H 1996 Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol 81: 521–527.
von Euler C, Trippenbach T 1976 Temperature effects on the inflation reflex during expiratory time in the cat. Acta Physiol Scand 96: 338–350.
Grunstein MM, Younes M, Milic-Emili J 1973 Control of tidal volume and respiratory frequency in anesthetized cats. J Appl Physiol 35: 463–476.
Bradley GW, von Euler C, Martilla I, Roos N 1974 Steady state effects of CO2 and temperature on the relationship between lung volume and inspiratory duration (Hering-Breuer threshold curve). Acta Physiol Scand 92: 351–363.
Widdicombe JG, Winning A 1974 Effects of hypoxia, hypercapnia and changes in body temperature on the pattern of breathing in cats. Respir Physiol 21: 203–221.
Schoener EP, Frankel HM 1972 Effect of hyperthermia and PaCO2 on the slowly adapting pulmonary stretch receptor. Am J Physiol 222: 68–72.
Bradley GW, Scheurmier N 1977 The transduction properties of tracheal stretch receptors in vitro. Respir Physiol 31: 365–375.
Loyola H, Fadic R, Cardenas H, Larrain C, Zapata P 1991 Effects of body temperature on chemosensory activity of the cat body in situ. Neurosci Lett 132: 251–254.
Alcayaga J, Sanhueza Y, Zapata P 1993 Thermal dependence of chemosensory activity in the carotid body superfused in vitro. Brain Res 600: 103–111.
Jansen AH, Chernick V 1983 Development of respiratory control. Physiol Rev 63: 437–483.
Finer NN, Abroms IF, Taeusch HW Jr 1976 Ventilation and sleep states in newborn infants. J Pediatr 89: 100–108.
Sullivan CE, Murphy E, Kozar LF, Phillipson EA 1979 Ventilatory responses to CO2 and lung inflation in tonic versus phasic REM sleep. J Appl Physiol 47: 1304–1310.
Stanton AN 1984 Overheating and cot death. Lancet 2: 1199–1201.
Sawczenko A, Fleming PJ 1996 Thermal stress, sleeping position, and the sudden infant death syndrome. Sleep 19:S267–S270.
Acknowledgements
The authors thank Lina Naso for the technical assistance, and Teresa Trippenbach for the critical reading of the manuscript.
Author information
Authors and Affiliations
Additional information
Supported by funds from the Medical Research Council of Canada. D.M. was supported by a bursary in Neonatology from the Fondo J. Miglierina, Varese, Italy, and was on leave from Hospital S. Anna, Como, Italy.
Rights and permissions
About this article
Cite this article
Merazzi, D., Mortola, J. Effects of Changes in Ambient Temperature on the Hering-Breüer Reflex of the Conscious Newborn Rat. Pediatr Res 45, 370–376 (1999). https://doi.org/10.1203/00006450-199903000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199903000-00014