Abstract
Inappropriate preparation of respiratory gases is associated with serious complications during mechanical ventilation. To develop a temperature monitoring system of respiratory gases within the endotracheal tube, four newborn piglets were studied using an ultra-rapid-response thermometer attached to the closed endotracheal tube suction system. Respiratory gas temperatures were monitored at the mouth-corner level of the endotracheal tube using three thermocouples (Tairway, inserted into the endotracheal tube via the closed suction system; Ttube_centre and Ttube_wall, embedded within the endotracheal tube 0.5 mm and 1.6 mm from the tube wall, respectively). Univariate analysis showed that inspiratory Ttube_centre and inspiratory Ttube_wall were positively correlated with inspiratory Tairway (both p < 0.001). Multivariate analysis showed the dependence of inspiratory Tairway on inspiratory Ttube_centre and Ttube_wall and deflation of endotracheal tube cuff (p < 0.001, p = 0.001 and p = 0.046, respectively). Inspiratory gas temperature within the endotracheal tube can be monitored using a thermometer attached to the closed endotracheal tube suction system. Our system, with further validation, might help optimise respiratory gas humidification during mechanical ventilation.
Similar content being viewed by others
Introduction
For patients who require mechanical ventilation, the inspired gas is heated and humidified to reduce drying of the respiratory mucosa, airway ulceration and impaired secretion clearance1,2,3. Insufficient and excessive humidification of respiratory gases are associated with serious complications, such as endotracheal tube obstruction and ventilator-associated pneumonia (VAP)1,2,4,5. The incidence rate of VAP is 0.0–4.4 per 1000 days of mechanical ventilation6, which increases the mortality rate by 13% compared to those who do not develop VAP7. To reduce such complications, the humidity of respiratory gases is prepared to 44 mgH2O/L, or the saturated vapour pressure at the body temperature of 37 °C, within the humidifying chamber8,9. Condensation is reduced by heating the respiratory gases to 40 °C before reaching the Y-piece, with the expectation that the gases will cool back down to 37 °C through the non-heated endotracheal tube.
However, the temperature reduction through the endotracheal tube varies according to the ambient temperature and several other settings10. Despite the importance of monitoring the humidity of respiratory gases provided to the patient, there is no established method for monitoring the humidity of respiratory gases at the distal end of the endotracheal tube, because non-invasive placement of hygrometers within the endotracheal tube is difficult. In addition, most hygrometers cannot distinguish inspiratory gas humidity from expiratory gas humidity because of their limited time constant.
Although direct monitoring of inspiratory gas humidity appears difficult, the humidity of gases delivered to the patient can be estimated using their lowest temperature before reaching the patient’s airway mucosa. Theoretically, this can be monitored at the distal end of the endotracheal tube at the mouth-corner level, because the gases are deprived of humidity from 44 mgH2O/L according to the extent of temperature drop below 37 °C.
We designed the current experimental study to test whether the inspiratory gas temperature can be monitored within the endotracheal tube at the mouth-corner level using an ultra-rapid-response thermometer, which is attached to the closed endotracheal tube suction system, in newborn piglets.
Results
Values are presented as the mean (standard deviation) unless otherwise indicated. Twelve temperature data collection cycles were performed from the four piglets (3 cycles per each); one cycle of which was excluded due to a malfunction of the humidifier. When Ttube_centre was expressed with time, the highest temperature values were observed at the beginning of the expiratory phase and the lowest during the inspiratory phase (Fig. 1).
Representative time-trend data of respiratory gas temperatures. A representative waveform recorded with the following ventilator settings: respiratory rate, 30/min; peak airway pressure, 15 cmH2O; inspiratory time, 0.5 s; positive end-expiratory pressure, 5 cmH2O; and with inflating the endotracheal tube cuff. Tairway and Ttube_centre showed similar waveforms between each other with distinct temperature changes between inspiration and expiration, whereas Ttube_wall showed no apparent temperature changes between inspiration and expiration. Exp. expiratory phase; Insp. inspiratory phase; Tairway airway temperature; Ttube_centre temperature at the centre of the endotracheal tube; and Ttube_wall temperature at the wall surface of the endotracheal tube.
Inspiratory temperature of three different locations
The average inspiratory temperature at the centre of the endotracheal tube (Ttube_centre), temperature at the wall surface of the endotracheal tube (Ttube_wall) and airway temperature (Tairway) were 38.6 (1.0)°C, 38.5 (0.9)°C and 39.4 (1.0)°C, respectively, whereas the average expiratory Ttube_centre, Ttube_wall and Tairway were 39.8 (1.0)°C, 38.6 (0.9)°C and 39.8 (0.9)°C, respectively (Fig. 2). (See the “Methods” section for the detail of the temperature measurement).
Temperature values recorded at three different locations. Tairway and Ttube_centre were relatively higher for the expiratory phase than for the inspiratory phase. Symbols: box, first and third quartiles; bold line, median; perpendicular line, range. Tairway airway temperature; Ttube_centre temperature at the centre of the endotracheal tube; and Ttube_wall temperature at the wall surface of the endotracheal tube.
Dependence of inspiratory Tairway on other temperature measures and ventilator settings
In the univariate analysis, inspiratory Ttube_centre and Ttube_wall were positively correlated with Tairway (both p < 0.001; see Supplementary Fig. S1 online). The peak inspiratory pressures (PIPs) of 15 and 20 cmH2O were associated with higher inspiratory Tairway (vs. PIP of 10 cmH2O, p = 0.013 and p = 0.004, respectively). Inspiratory Tairway did not depend on the difference in the inspiratory time and inflation of endotracheal tube cuff. The multivariate analysis showed that higher inspiratory Tairway was best explained by higher inspiratory Ttube_centre, higher inspiratory Ttube_wall and deflation of endotracheal tube cuff (p < 0.001, p = 0.001 and p = 0.046, respectively; Table 1).
Discussion
Our data from newborn piglets suggested that the inspiratory temperature of respiratory gases can be monitored using an ultra-rapid-response thermometer attached to the closed endotracheal tube suction system. With further validation, our airway thermometer might help optimise the humidity level of respiratory gases for mechanically ventilated patients and prevent complications associated with inappropriate humidification.
Appropriate heating and humidification of respiratory gases are important to prevent complications during mechanical ventilation7. Previous studies addressed the respiratory gas humidity using dummy respiratory circuits and artificial lungs11,12,13. However, there currently is no established method to monitor the temperature and humidity of respiratory gases at the distal end of the endotracheal tube. Haugk and colleagues proposed that periodic changes in the temperature measured on the outer surface of the endotracheal tube cuff might help predict the inspiratory gas temperature14. However, in our study, even Ttube_wall, which was measured at the inner surface of the endotracheal tube, did not show significant temperature changes between inspiratory and expiratory phases, suggesting that the precise estimation of the inspiratory airway temperature would be difficult when the temperature is measured outside the endotracheal tube. Schiffmann and colleagues measured the respiratory gas humidity at the proximal end of the endotracheal tube using a specially designed hygrometer (sampling frequency, 20 Hz; time constant, unknown), which enabled the extraction of the inspiratory gas humidity from the expiratory gas humidity15. However, the inspiratory gas humidity at the proximal part of the endotracheal tube is clinically much less informative compared to that monitored at the distal end of the endotracheal tube10. In our study, we developed an airway thermometer by combining ultra-rapid-response thermocouples and a closed endotracheal tube suction system, which are commercially available and commonly used even in newborn infants16,17,18. By inserting a similar device into the endotracheal tube, temperature values which reflect the respiratory gas temperature at the mouth-corner level of the endotracheal tube, were monitored. Using the information provided by the manometer of the ventilator, we were able to identify the inspiratory and expiratory temperatures independently of each other. Although we used the simultaneous video recording to the graphic monitor of the ventilator, this information might be unnecessary because both Tairway and Ttube_centre showed distinct patterns between inspiratory and expiratory phases when the temperature values were plotted along time. However, considering that expiratory gas temperatures at the mouth-corner level theoretically mirror core body temperatures, lower body temperatures may be associated with smaller differences between inspiratory and expiratory gas temperatures, where the information from the manometer might be necessary to precisely identify the inspiratory phase. In addition, we initially expected that expiratory Ttube_centre reflects the core body temperature. However, expiratory Ttube_centre was higher than 38.5 °C, to which the target rectal temperature was servo-controlled. It is possible that the true core body temperature was much higher than the rectal temperature, because the radiant heat was mainly focused on the trunk of the piglet, and the rectal temperature is relatively lower than the oesophageal temperature in newborn species19.
We did not measure the airway temperature at the distal end of the endotracheal tube. This is based on an assumption that the inspiratory gas temperature theoretically goes down when travelling from the Y-piece to the mouth-corner level of the endotracheal tube, because the respiratory gases are cooled by the ambient air (26 °C in the current study), and this temperature level is much lower than the respiratory gas temperature at the Y-piece (set to 40 °C). Once the respiratory gases have passed the mouth-corner level, they are heated back to the body temperature (38.5 °C in newborn piglets and 37 °C in newborn infants)20,21. Therefore, the saturated vapour pressure calculated from the inspiratory gas temperature measured at the mouth-corner level (but not at the distal end of the endotracheal tube) would be suitable to estimate the humidity level delivered to the patient’s bronchus.
Given the relatively non-invasive nature of the device, our airway thermometer is likely to provide crucial information to prevent complications of invasive ventilation, which are related to the inappropriate humidification of respiratory gases. Latent but inappropriate humidification of critical levels have been reported in patients who are undergoing therapeutic hypothermia22. The optimal humidifier setting has not been established when the body temperature is controlled other than normothermic23. The temperature and humidity of inspiratory gases remain largely unknown when heat-moist exchangers are used instead of active humidifying systems24. Monitoring of the airway temperature at the mouth-corner level may help optimise the humidifier setting for such conditions as well as many others, where the risk of inappropriate humidification and subsequent problems are increased. However, caution is required because Tairway is unlikely to reflect humidity levels of inspiratory gases when respiratory gases have not been fully saturated at the outlet of the humidifying chamber25.
Our study simultaneously showed the possible limitations of our airway thermometer, which need to be addressed in future studies. First, the relationship between Tairway and Ttube_centre is likely to be affected by the presence of an endotracheal tube leakage. Indeed, deflation of the cuff was associated with higher Tairway presumably due to increased inspiratory gas flow, which is required to maintain the airway pressure. Second, because of technical reasons, the body temperature of the piglet was maintained at the normal body temperature for piglets, which is higher than that for human newborn infants. Third, due to ethical reasons, we were only able to study four piglets. Hence, we were able to assess the impact of limited clinical variables on the temperature reading of the airway thermometer. To compensate for the lack of a sufficient subject number, we tried to maximise the variation of the ventilator settings. We also repeated the temperature data acquisition with adequate time intervals, the influence of which was accounted for using the generalised estimating equation. Fourth, inspiratory gas temperatures were higher for Tairway than Ttube_centre, suggesting the possibility that Ttube_centre was affected by the thermal exchange with the endotracheal tube. If this is correct, the true inspiratory gas temperature at the mouth corner level might better be reflected by Tairway rather than Ttube_centre. Fifth, we were unable to incorporate TY-piece values within the analysis because of the technical problem, although we did not expect TY-piece to be a good surrogate for Ttube_centre. Finally, all temperature data acquisitions were performed with spontaneous breathing attenuated by intravenous injections of a muscle relaxant. Therefore, the influence of spontaneous breathing needs to be assessed in future studies. Further preclinical and clinical studies are needed to validate the accuracy of the airway temperature estimation using the airway thermometer.
In newborn piglets, the inspiratory gas temperature travelling through the mouth-corner level of the endotracheal tube was successfully measured using an ultra-rapid-response thermometer attached to the closed endotracheal tube suction system. Using the measured temperature value, the condensation and humidity of the respiratory gases delivered to the bronchus of the newborn piglet can be calculated. With further validation, this relatively non-invasive airway thermometer may be useful for monitoring and optimising the humidity level of respiratory gases, and may contribute to the reduction of ventilation-associated complications. Further studies are required to validate the estimation of the inspiratory gas temperature in preclinical and clinical settings, where the influence of endotracheal tube leakage and spontaneous breathing needs to be assessed.
Methods
Study subjects and animal preparation
The protocol of this study was approved by the animal ethics committee of the Institute of Animal Experimentation, Kurume University School of Medicine, Fukuoka, Japan. The study was conducted in accordance with the principles of the Declaration of Helsinki. All the experiments were performed in compliance with the ARRIVE guidelines26. Four male newborn piglets (2 to 3 days old, 1.2 to 3.4 kg) were studied within 72 h of birth. The piglets were delivered from the farm in the morning of the experiment and sedated with an intramuscular injection of midazolam (0.2 mg/kg). Each piglet was placed on an open incubator and a peripheral venous catheter was inserted to give maintenance fluid (10% glucose, 80 ml/kg/day) and anaesthetics. The room was air-conditioned to maintain the ambient temperature of 26 °C. The electrocardiogram and percutaneous arterial oxygen saturation were monitored continuously, whereas non-invasive blood pressure and blood gases were assessed approximately every 30 min. General anaesthesia was then induced by 5% sevoflurane using a mask, and the piglet was intubated using a cuffed endotracheal tube with the internal diameter of 3 mm. The piglet was then placed in the left-lateral position and mechanically ventilated to maintain normal PCO2 using the pressure-control ventilation mode. Anaesthesia was maintained by 2–5% inhaled sevoflurane, a continuous intravenous infusion of dexmedetomidine (1 μg/kg/h) and intravenous pancuronium injections (0.7 mg/kg/dose, approximately every 30 min). The body temperature of the piglet was maintained at 38.5 ± 0.5 °C using a radiant heater, which was servo-controlled with rectal temperature feedback.
Temperature monitoring
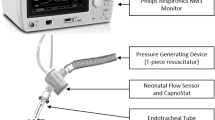
A custom-made airway thermometer was developed using an ultra-fast-response thermocouple (time constant, 0.05 ms; FasTemp, Aivision, Tokyo, Japan) at the distal tip of the closed suction system (Ecocath, Medtronic plc., Dublin, Ireland). The respiratory gas temperature at the Y-piece of the ventilator circuit was monitored using a built-in thermometer of the humidifying system (MR 730, Fisher and Paykel, Auckland, New Zealand) and another FasTemp probe (TY-piece). To assess the accuracy of the temperature measured using the airway thermometer (Tairway), another custom-made system was developed by attaching two FasTemp probes to an 8 mm long tube with an internal diameter of 3.2 mm, 1.6 mm (Ttube_centre) and 0.5 mm (Ttube_wall) from the endotracheal tube wall to the centre of the endotracheal tube (Fig. 3); this unit was inserted into an endotracheal tube (internal diameter, 3 mm) once the animal was intubated and the endotracheal tube was cut at the level of the mouth (Fig. 4).
Diagram demonstrating the construction of an extension part of the endotracheal tube to monitor the respiratory gas temperature at the centre and the wall part of the tube. An extension part of the endotracheal tube was constructed by attaching two ultra-fast-response thermocouples [1.6 mm (Ttube_centre) and 0.5 mm (Ttube_wall)] from the endotracheal tube wall toward the centre of the endotracheal tube (a,b). This extension part was inserted between the distal and proximal parts of the endotracheal tube at the level of the mouth (c,d). Ttube_centre temperature at the centre of the endotracheal tube; and Ttube_wall temperature at the wall surface of the endotracheal tube.
Diagrams depicting the configuration of the ventilator circuit and thermometers. An ultra-fast-response thermocouple was attached to the distal end of the closed suction system. Respiratory gas temperatures were measured using an airway thermometer (Tairway) and other ultra-fast-response thermocouples attached to the Y-piece (TY-piece) and the extension part of the endotracheal tube (see Fig. 3 for details).
Prior to the experiment, each FasTemp probe was inspected so that temperature values are within ± 0.3 °C of the standard mercury thermometer using a water bath of 30 to 40 °C. Electronic signals from the thermocouples were transferred to a laptop computer through a data logger (C-Logger, Contec, Osaka, Japan) with a sampling rate of 200 Hz.
Ventilators, circuits and humidifiers
We used a ventilator (Servo-300 ventilator, Siemens Elema, Solna, Sweden) with a humidifier and a disposable circuit (DAR Neonatal, Medtronic, Dublin, Ireland) which were configured based on the manufacturer’s recommendation (Fig. 4).
Data collection
Temperature information was collected with the clocks of the data logging system, video camera and ventilator synchronised between each other prior to the experiment. Data acquisition was started before the insertion of the airway thermometer into the level of the mouth-corner, where the airway thermometer was withheld for at least 30 s, and was terminated after the removal of the airway thermometer (see Supplementary Fig. S2 online). Data acquisition was performed for a set of conditions with six different ventilator settings [combinations of PIPs (10, 15 and 20 cmH2O) and inspiratory times (0.5 and 1.0 s)] and with/without inflating the endotracheal tube cuff with 2.5 ml of air (cuff pressure was not measured since tracheal safety was not an issue in the current experiment); the pressure–volume curve of the ventilator graphic monitor was inspected to confirm that leakage at cuff/trachea interface was successfully eliminated by the cuff inflation.
The positive end-expiratory pressure (PEEP) and ventilation frequency were fixed to 5 cmH2O and 30 /min, respectively, whereas the humidifying chamber outlet and Y-piece temperatures were set to 37 °C and 40 °C, respectively. The data collection cycle (see Supplementary Fig. S1 online) was repeated up to three times with intervals of at least 30 min from the same piglet. After the completion of the study, piglets were euthanised by an overdose injection of intravenous phenobarbital.
Data analysis
The time-trend data were assessed on a spreadsheet (Excel, Microsoft, DC, U.S.A.; MATLAB, Math Works, MA, U.S.A.) and classified into inspiratory and expiratory phases referring to the video images of the graphic monitor. Five breaths were chosen from the 30 s record per each ventilator setting to identify the highest expiratory temperature and lowest inspiratory temperature for Tairway, Ttube_centre and Ttube_wall, excluding 5 breaths immediately before and after ventilator setting changes. We also tried to extract inspiratory and expiratory TY-piece, however, because of the intermittent high-frequency digital noise observed for the data from this channel, we decided not to consider TY-piece further.
The average of the five temperature values for each ventilator setting was used for the analysis. Independent variables to explain inspiratory Tairway were assessed using Ttube_centre, Ttube_wall, peak inspiratory pressure (10, 15 and 20 cmH2O), inspiratory time and the use of the balloon cuff (inflation and deflation). Univariate and multivariate generalised estimating equations were used to account for repeated sampling from the same piglet. This model was assessed with the Wald Chi-square test statistic with a level of significance set at p < 0.05.
References
Williams, R., Rankin, N., Smith, T., Galler, D. & Seakins, P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit. Care Med. 24, 1920–1929 (1996).
Branson, R. D. The effects of inadequate humidity. Respir. Care. Clin. N. Am. 4, 199–214 (1998).
Solomita, M., Daroowalla, F., Leblanc, D. S. & Smaldone, G. C. Y-piece temperature and humidification during mechanical ventilation. Respir. Care. 54, 480–486 (2009).
Pillow, J. J. et al. Oxygen, temperature and humidity of inspired gases and their influences on airway and lung tissue in near-term lambs. Intensive Care Med. 35, 2157–2163 (2009).
Sottiaux, T. M. Consequences of under- and over-humidification. Respir. Care. Clin. N. Am. 12, 233–252 (2006).
Dudeck, M. A. et al. National Healthcare Safety Network (NHSN) report, data summary for 2012 Device-associated module. Am. J. Infect. Control. 41, 1148–1166 (2013).
Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13, 665–671 (2013).
ISO. 80601-2-74. ISO; 2017. Medical Electrical Equipment—Part 2–74: Particular Requirements for Basic Safety and Essential Performance of Respiratory Humidifying Equipment. https://www.iso.org/standard/65008.html. (2017).
Restrepo, R. D., Walsh, B. K. & Care AAfR. Humidification during invasive and noninvasive mechanical ventilation: 2012. Respir Care. 57, 782–788 (2012).
Yamada, Y. et al. A drop in gas temperature in the external part of the endotracheal tube is problematic during neonatal respiratory support. Pediatr. Pulmonol. 43, 666–673 (2008).
Jardine, L. A., Dunster, K. R. & Davies, M. W. An experimental model for the measurement of inspired gas temperatures in ventilated neonates. Pediatr. Pulmonol. 43, 29–33 (2008).
Haziot, N. et al. Impact of leaks and ventilation parameters on the efficacy of humidifiers during home ventilation for tracheostomized patients: A bench study. BMC Pulm. Med. 19, 43 (2019).
Chikata, Y., Imanaka, H., Onishi, Y., Ueta, M. & Nishimura, M. Humidification during high-frequency oscillation ventilation is affected by ventilator circuit and ventilatory setting. Paediatr. Anaesth. 19, 779–783 (2009).
Haugk, M. et al. Temperature monitored on the cuff surface of an endotracheal tube reflects body temperature. Crit. Care Med. 38, 1569–1573 (2010).
Schiffmann, H. et al. Airway humidification in mechanically ventilated neonates and infants: A comparative study of a heat and moisture exchanger vs. a heated humidifier using a new fast-response capacitive humidity sensor. Crit. Care Med. 25, 1755–1760 (1997).
Cereda, M. et al. Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med. 27, 648–654 (2001).
Care AAfR. AARC Clinical Practice Guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir. Care. 55, 758–764 (2010).
Tan, A. M. et al. Closed versus partially ventilated endotracheal suction in extremely preterm neonates: Physiologic consequences. Intensive Crit. Care Nurs. 21, 234–242 (2005).
Sarkar, S., Donn, S. M., Bhagat, I., Dechert, R. E. & Barks, J. D. Esophageal and rectal temperatures as estimates of core temperature during therapeutic whole-body hypothermia. J. Pediatr. 162, 208–210 (2013).
Nuntapaitoon, M., Muns, R. & Tummaruk, P. Newborn traits associated with pre-weaning growth and survival in piglets. Asian-Australas J. Anim. Sci. 31, 237–244 (2018).
Fransson, A. L., Karlsson, H. & Nilsson, K. Temperature variation in newborn babies: Importance of physical contact with the mother. Arch. Dis. Child. Fetal Neonatal Ed. 90, 500–504 (2005).
Tanaka, S. et al. Use of normothermic default humidifier settings causes excessive humidification of respiratory gases during therapeutic hypothermia. Ther. Hypothermia Temp. Manag. 6, 180–188 (2016).
Tanaka, Y. et al. Insufficient humidification of respiratory gases in patients who are undergoing therapeutic hypothermia at a paediatric and adult intensive care unit. Can. Respir. J. 2017, 8349874 (2017).
Matteo, F. et al. A new method for in vivo analysis of the performances of a heat and moisture exchanger (HME) in mechanically ventilated patients. Pulm. Med. 2019, 9270615 (2019).
Lellouche, F. et al. Influence of ambient and ventilator output temperatures on performance of heated-wire humidifiers. Am. J. Respir. Crit Care Med. 170, 1073–1079 (2004).
Carol, K., William, J. B., Innes, C. C., Michael, E. & Douglas, G. A. Improving bioscience reserch reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8, 1000412. https://doi.org/10.1371/journal.pbio.1000412 (2010).
Acknowledgements
The authors thank Drs. Yasuhisa Nakamura, Takenori Kato and Tadashi Hisano for their assistance with the data collection. This work was supported by the Japan Society for the Promotion of Science, The Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research 18K07795, 17K16286 and 16K09005).
Author information
Authors and Affiliations
Contributions
S.N., M.K. and O.I. designed the study protocol. K.T. and M.K. performed and recorded animal experiments. S.N. and O.I. performed the statistical analyses and contributed to interpretation of findings. S.N. prepared the figures and drafted the initial manuscript. K.T., M.K., S.K., S.I., Y.L., M.M., S.S. and O.I. critically reviewed and revised the manuscript. All authors have seen and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakane, S., Tsuda, K., Kinoshita, M. et al. Airway gas temperature within endotracheal tube can be monitored using rapid response thermometer. Sci Rep 11, 9537 (2021). https://doi.org/10.1038/s41598-021-88787-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88787-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.