Abstract
Background
Significant cardiorespiratory events can be triggered in preterm infants as part of laryngeal chemoreflexes (LCRs) and esophageal reflexes (ERs). We previously showed that nasal continuous positive airway pressure (nCPAP) blunted the cardiorespiratory inhibition induced with LCRs. Therefore, we aimed to compare the effects of nCPAP and high-flow nasal cannulas (HFNC) on the cardiorespiratory events induced during LCRs and ERs. The hypothesis is that nCPAP but not HFNC decreases the cardiorespiratory inhibition observed during LCRs and ERs.
Methods
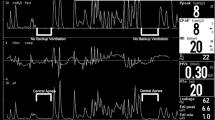
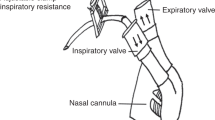
Eleven preterm lambs were instrumented to record respiration, ECG, oxygenation, and states of alertness. LCRs and ERs were induced during non-rapid eye movement sleep in a random order under these conditions: nCPAP 6 cmH2O, HFNC 7 L/min, high-flow nasal cannulas 7 L/min at a tracheal pressure of 6 cmH2O, and no respiratory support.
Results
nCPAP 6 cmH2O decreased the cardiorespiratory inhibition induced with LCRs, but not with ERs in preterm lambs. This blunting effect was less marked with HFNC 7 L/min, even when the tracheal pressure was maintained at 6 cmH2O.
Conclusions
nCPAP might be a treatment for cardiorespiratory events related to LCRs in newborns, either in the context of laryngopharyngeal refluxes or swallowing immaturity. Our preclinical results merit to be confirmed through clinical studies.
Impact
-
Laryngeal chemoreflexes can be responsible for significant cardiorespiratory inhibition in newborns, especially preterm.
-
Nasal continuous positive airway pressure at 6 cmH2O significantly decreased this cardiorespiratory inhibition.
-
High-flow nasal cannulas at 7 L/min had a lesser effect than nasal continuous positive airway pressure.
-
Esophageal stimulation was responsible for a smaller cardiorespiratory inhibition, which was not significantly modified by nasal continuous positive airway pressure or high-flow nasal cannulas.
-
Nasal continuous positive airway pressure should be tested for its beneficial effect on cardiorespiratory events related to laryngeal chemoreflexes in preterm newborns.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study is included in this published article [and its supplementary information files].
References
Hasenstab, K. A. & Jadcherla, S. R. Gastroesophageal reflux disease in the neonatal intensive care unit neonate: controversies, current understanding, and future directions. Clin. Perinatol. 47, 243–263 (2020).
Praud, J. P., Cornerstone, A. & Kemp, J. In Kendig’s Disorders of the Respiratory Tract (eds. Willmott, R. W. et al.) (Elsevier Saunders, 2023).
St-Hilaire, M. et al. Postnatal maturation of laryngeal chemoreflexes in the preterm lamb. J. Appl. Physiol. (1985) 102, 1429–1438 (2007).
Thach, B. T. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. Am. J. Med. 103, 120S–124S (1997).
Cresi, F. et al. Cardiorespiratory events in infants with gastroesophageal reflux symptoms: is there any association? Neurogastroenterol. Motil. 30, e13278 (2018).
Hasenstab, K. A., Nawaz, S., Lang, I. M., Shaker, R. & Jadcherla, S. R. Pharyngoesophageal and cardiorespiratory interactions: potential implications for premature infants at risk of clinically significant cardiorespiratory events. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G304–G312 (2019).
Nault, S., Samson, N., Nadeau, C., Djeddi, D. & Praud, J. P. Reflex cardiorespiratory events from esophageal origin are heightened by preterm birth. J. Appl. Physiol. (1985) 123, 489–497 (2017).
Jadcherla, S. R. et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am. J. Gastroenterol. 103, 720–728 (2008).
Thach, B. T. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am. J. Med. 111, 69S–77S (2001).
Schey, W. L., Replogle, R., Campbell, C., Meus, P. & Levinsky, R. A. Esophageal dysmotility and the sudden infant death syndrome: clinical experience. Radiology 140, 67–71 (1981).
Kuypers, K. et al. Reflexes that impact spontaneous breathing of preterm infants at birth: a narrative review. Arch. Dis. Child. Fetal Neonatal Ed. 105, 675–679 (2020).
Uchiyama, A., Ochiai, S. & Murayama, Y. Noninvasive respiratory support following extubation in preterm infants. Pediatr. Int. 65, e15535 (2023).
Dani, C. Nasal continuous positive airway pressure and high-flow nasal cannula today. Clin. Perinatol. 48, 711–724 (2021).
Hong, H., Li, X. X., Li, J. & Zhang, Z. Q. High-flow nasal cannula versus nasal continuous positive airway pressure for respiratory support in preterm infants: a meta-analysis of randomized controlled trials. J. Matern. Fetal Neonatal Med. 34, 259–266 (2021).
Nasef, N. et al. High-flow nasal cannulae are associated with increased diaphragm activation compared with nasal continuous positive airway pressure in preterm infants. Acta Paediatr. 104, e337–e343 (2015).
Boudaa, N. et al. Effects of caffeine and/or nasal CPAP treatment on laryngeal chemoreflexes in preterm lambs. J. Appl. Physiol. (1985) 114, 637–646 (2013).
Djeddi, D., Cantin, D., Samson, N. & Praud, J. P. Nasal continuous positive airway pressure inhibits gastroesophageal reflux in newborn lambs. PLoS One 9, e107736 (2014).
Elsedawi, B. F. et al. Safety of bottle-feeding under nasal respiratory support in preterm lambs with and without tachypnoea. Front. Physiol. 12, 785086 (2022).
Alain, C. et al. Nasal respiratory support and tachypnea and oral feeding in full-term newborn lambs. J. Appl. Physiol. (1985) 130, 1436–1447 (2021).
Samson, N., Dumont, S., Specq, M. L. & Praud, J. P. Radio telemetry devices to monitor breathing in non-sedated animals. Respir. Physiol. Neurobiol. 179, 111–118 (2011).
Specq, M. L. et al. Moderate hyperbilirubinemia alters neonatal cardiorespiratory control and induces inflammation in the nucleus tractus solitarius. Front. Physiol. 7, 437 (2016).
Renolleau, S., Letourneau, P., Niyonsenga, T., Praud, J. P. & Gagne, B. Thyroarytenoid muscle electrical activity during spontaneous apneas in preterm lambs. Am. J. Respir. Crit. Care Med. 159, 1396–1404 (1999).
Kato, I. et al. Incomplete arousal processes in infants who were victims of sudden death. Am. J. Respir. Crit. Care Med. 168, 1298–1303 (2003).
St-Hilaire, M. et al. Laryngeal chemoreflexes induced by acid, water, and saline in nonsedated newborn lambs during quiet sleep. J. Appl. Physiol. (1985) 98, 2197–2203 (2005).
Bourgoin-Heck, M. et al. Effects of moderate hyperbilirubinemia on nutritive swallowing and swallowing-breathing coordination in preterm lambs. Neonatology 108, 42–48 (2015).
Praud, J. P. Upper airway reflexes in response to gastric reflux. Paediatr. Respir. Rev. 11, 208–212 (2010).
Berridge, C. W., Schmeichel, B. E. & Espana, R. A. Noradrenergic modulation of wakefulness/arousal. Sleep. Med. Rev. 16, 187–197 (2012).
Filiano, J. J. & Kinney, H. C. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol. Neonate 65, 194–197 (1994).
Abu Jawdeh, E. G. & Martin, R. J. Neonatal apnea and gastroesophageal reflux (Ger): is there a problem? Early Hum. Dev. 89, S14–S16 (2013).
Di Fiore, J. M., Arko, M., Whitehouse, M., Kimball, A. & Martin, R. J. Apnea is not prolonged by acid gastroesophageal reflux in preterm infants. Pediatrics 116, 1059–1063 (2005).
Poets, C. F. Interventions for apnoea of prematurity: a personal view. Acta Paediatr. 99, 172–177 (2010).
Rossor, T., Andradi, G., Ali, K., Bhat, R. & Greenough, A. Gastro-oesophageal reflux and apnoea: is there a temporal relationship? Neonatology 113, 206–211 (2018).
Jadcherla, S. R. et al. Impact of feeding strategies with acid suppression on esophageal reflexes in human neonates with gastroesophageal reflux disease: a single-blinded randomized clinical trial. Clin. Transl. Gastroenterol. 11, e00249 (2020).
Sankaran, J., Qureshi, A. H., Woodley, F., Splaingard, M. & Jadcherla, S. R. Effect of severity of esophageal acidification on sleep vs wake periods in infants presenting with brief resolved unexplained events. J. Pediatr. 179, 42–48.e41 (2016).
Ramet, J. et al. Cardiac and respiratory responses to esophageal dilatation during rem sleep in human infants. Biol. Neonate 58, 181–187 (1990).
Ramet, J. Cardiac and respiratory reactivity to gastroesophageal reflux: experimental data in infants. Biol. Neonate 65, 240–246 (1994).
Kenigsberg, K., Griswold, P. G., Buckley, B. J., Gootman, N. & Gootman, P. M. Cardiac effects of esophageal stimulation: possible relationship between gastroesophageal reflux (Ger) and sudden infant death syndrome (Sids). J. Pediatr. Surg. 18, 542–545 (1983).
Jadcherla, S. R. et al. Effect of nasal noninvasive respiratory support methods on pharyngeal provocation-induced aerodigestive reflexes in infants. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G1006–G1014 (2016).
Jadcherla, S. R., Hoffmann, R. G. & Shaker, R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J. Pediatr. 149, 77–82 (2006).
Kinkead, R. & Schlenker, E. Sex-based differences in respiratory control: progress in basic physiology and clinical research. Respir. Physiol. Neurobiol. 245, 1–3 (2017).
Kouchi, H., Uppari, N., Joseph, V., & Bairam, A. Sex-specific respiratory effects of acute and chronic caffeine administration in newborn rats. Respir. Physiol. Neurobiol. 240, 8–16 (2017).
Rousseau, J. P. et al. On the origins of sex-based differences in respiratory disorders: lessons and hypotheses from stress neuroendocrinology in developing rats. Respir. Physiol. Neurobiol. 245, 105–121 (2017).
Baldy, C., Chamberland, S., Fournier, S. & Kinkead, R. Sex-specific consequences of neonatal stress on cardio-respiratory inhibition following laryngeal stimulation in rat pups. eNeuro 4, ENEURO.0393-17.2017 (2017).
Di Fiore, J. M., Poets, C. F., Gauda, E., Martin, R. J. & MacFarlane, P. Cardiorespiratory events in preterm infants: interventions and consequences. J. Perinatol. 36, 251–258 (2016).
Erickson, G., Dobson, N. R. & Hunt, C. E. Immature control of breathing and apnea of prematurity: the known and unknown. J. Perinatol. 41, 2111–2123 (2021).
Thach, B. T. The role of respiratory control disorders in sids. Respir. Physiol. Neurobiol. 149, 343–353 (2005).
Hunt, C. E. Ontogeny of autonomic regulation in late preterm infants born at 34–37 weeks postmenstrual age. Semin. Perinatol. 30, 73–76 (2006).
Funding
This study was supported by the Canada Research Chair in Neonatal Respiratory Physiology and an operating grant (CIHR #15558) from the Canadian Institutes of Health Research allocated to J.P.P. E.F.P. and J.P.P. are members of the Sherbrooke University Hospital Research Center, the Quebec Respiratory Health Research Network, and the Quebec Research Network on Perinatal Determinants of Children Health.
Author information
Authors and Affiliations
Contributions
N.S., C.N., E.F.P., and J.P.P. conceived the research and designed the protocol. N.S. and C.N. performed the deliveries. B.F.E., N.S., C.N., A.C., and A.L. ensured the care of the preterm lambs. B.F.E., N.S., and C.N. performed animal experiments. B.F.E., N.S., C.N., and A.C. analyzed the data. B.F.E., N.S., E.F.P., and J.P.P. interpreted the results. B.F.E. prepared the figures and wrote the first draft of the manuscript. N.S. and J.P.P. revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elsedawi, B.F., Samson, N., Nadeau, C. et al. Effects of Nasal Respiratory Support on Laryngeal and Esophageal Reflexes in Preterm Lambs. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02883-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02883-w
This article is cited by
-
Should nasal respiratory support in preterm infants be tailored for physiologic maturity?
Pediatric Research (2024)