Abstract
Radioresistance, which is a major cause of failure of radiotherapy (RT), is proposed as one of the intrinsic characteristics of cancer stem cells (CSCs) whose unique DNA damage response (DDR), efficient DNA repair and resistance to apoptosis are thought to confer the phenotype. We have isolated surviving CSCs by exposure to long-term fractionated radiation for 82 days from HepG2 and A172 cells (82FR-31NR cells). 82FR-31NR cells exhibited CSC properties, such as high expression of CSC marker CD133 and the ABC transporters (MDR1 and BCRP1), and high tumorigenic potential after transplantation into nude mice. The advantage of our isolated CSCs is that they can proliferate in as the same growth medium as that of parental cells without loss of CSC properties. Therefore, we can analyze DDR of non-stem cells and CSCs without any influences caused by different culture conditions. 82FR-31NR cells showed efficient DNA repair of radiation-induced DNA damage and radioresistance with activation of the AKT/cyclin D1 survival signaling pathway. In contrast, DNA damage persisted for a long time after irradiation in parental cells compared with isolated CSCs. Persisted DNA damage induced apoptosis in parental cells without activation of the AKT/cyclin D1 pathway. Therefore, inhibition of the AKT/cyclin D1 pathway by an AKT inhibitor, API-2, or cyclin D1 siRNA resulted in a loss of efficient DNA repair and radiosensitization of 82FR-31NR cells. Furthermore, knockdown of Cdk4 by its siRNA or a Cdk4 inhibitor was sufficient to suppress radioresistance of CSCs. In this study, we present a newly discovered DDR regarding the AKT/cyclin D1/Cdk4 pathway in response to radiation in CSCs. Combination of fractionated RT and reagents targeting the AKT/cyclin D1/Cdk4 pathway to eradicate CSCs would be effective therapeutic modality.
Similar content being viewed by others
Introduction
Radiotherapy (RT) is one of the major modalities of cancer treatment with excellent tumor control, preservation of normal tissues and less systemic influences. General protocol of fractionated RT consists of daily exposures to a fraction dose of around 2 Gy for 5–7 weeks. Although tumors receive a large total dose by multiple fractionated radiation (FR), they sometimes recur with radioresistance. Repopulation of surviving tumor cells during fractionated RT limits the efficacy of RT and is the major cause of failure of RT.1 Tumors consist of a heterogeneous population of cells that contain a subpopulation of cancer stem cells (CSCs) that are defined by the capacity of self-renewal and the generation of heterogeneous lineages of cancer cells.2 Tumor radioresistance is thought to be caused by CSCs, which harbor preferential activation of the DNA damage response (DDR), efficient DNA repair machinery and resistance to apoptosis.3, 4, 5, 6 Therefore, targeting CSCs is likely to be the key to cure cancer for the development of more effective fractionated RT.7 Despite recent progress in CSC study, our knowledge of the molecular target for suppressing radioresistance of CSCs remains to be answered.
Stem cell medium containing epidermal growth factor and basic fibroblast growth factor without serum is required for in vitro culture of CSCs. The PI3K/AKT pathway is activated in stem cell medium, which is important in the maintenance and survival of CSCs in vitro.8, 9, 10 Accumulating evidence suggests that the PI3K/AKT signaling pathway is a major contributor to tumor radioresistance. Active AKT, a common mediator of cell survival signals induced by radiation through multiple intracellular signaling pathways,11, 12 suppresses apoptosis. AKT positively regulates cyclin D1 expression through inactivation of glycogen synthase kinase 3β (GSK3β). The AKT-mediated phosphorylation of glycogen synthase kinase 3β on serine9 decreases its kinase activity for Thr286 of cyclin D1, which inhibits the nuclear export and the cytoplasmic proteasomal degradation of cyclin D1.13, 14 Thus, the activation of the AKT pathway leads to nuclear accumulation of cyclin D1 resulting in cell proliferation. Overexpression of cyclin D1 is strongly correlated with the poor prognosis of oral, and head and neck squamous cell carcinoma after RT or chemo-RT. Cyclin D1 is considered as a therapeutic target for these cancers.15 We have previously reported that the AKT/glycogen synthase kinase 3β/cyclin D1 pathway is implicated in acquired radioresistance of tumor cells triggered by long-term FR.16 Targeting the pathway completely suppressed tumor regrowth after FR in vivo.17 However, the precise role of AKT/cyclin D1 pathway in radioresistance of CSCs remains to be elucidated.
In this study, we have isolated CD133-positive CSCs from HepG2 and A172 by exposure to FR for 82 days. These cells showed radioresistance with the activation of the AKT/cyclin D1 pathway following irradiation, but this activation was not observed in corresponding parental cells. Radioresistance of CSCs was suppressed by combination of 2 Gy of FR and reagents such as an AKT inhibitor of AKT/PKB signaling pathway (API-2) and a Cdk4 inhibitor. Thus, we have demonstrated that AKT, cylin D1 and Cdk4 are important molecular targets to suppress radioresistance of CSCs.
Results
Isolation of CD133-positive CSCs by long-term FR
In order to isolate radioresistant tumor cells against long-term FR, HepG2 and A172 cells were exposed to FR with 0.5 Gy of X-rays every 12 h for up to 82 days. A dramatic increase in the level of CD133, a marker of CSCs18 was observed after FR for 82 days in all cell lines examined, but was not in cells exposed for 14 days (14FR cells) and 31 days (31FR cells) (Figure 1a). We have previously reported that 31FR cells have acquired radioresistance by exposure to FR for 31 days.16 31FR cells with acquired radioresistance are not equal to CSCs because they were negative for CD133 (Figure 1a). We next measured the ratio of CD133-positive cells by fluorescence-activated cell sorting (FACS) with phycoerythrin-conjugated anti-CD133 antibody in 82FR cells after the cessation of FR for over 1 month (hereafter referred to as 82FR-31 non-radiation (82FR-31NR) cells) (Figure 1b). CD133-positive cells were <10% before FR, but increased to around 90% in 82FR-31NR cells in both cell lines (Figure 1c).
Isolation of CD133-positive cells by radiation exposure. (a) Western blotting of CD133 and β-actin in HepG2 and A172 cells at the indicated day of FR. (b) FACS analysis of CD133-positive cells by using phycoerythrin-conjugated anti-CD133 antibodies. Phycoerythrin-conjugated mouse-IgG antibody is used as negative control. (c) The percentage of CD133-positive cells was determined by FACS analysis as shown in b. (d) CD133 positive cells in the colony of HepG2. The colony was made by incubating irradiated cells for 6 days and then immunofluorescence was performed. The percentage of the colony consisting of CD133-positive cells was shown on the right graph. (e) mRNA expression of GADPH, CD133, Albumin, MDR1, GFAP and BCRP1 in parental 0FR cells (lane 1) and 82FR-31NR cells (lane 2). GADPH was used for internal control. (f) Immunofluorescence staining with cytokeratin14 in 0FR and 82FR-31NR cells of HepG2. Nuclei were stained with Hoechst.
CD133-positive cells were likely to be enriched by selectively killing radiosensitive CD133-negative cells by FR. In order to examine this possibility, CD133 expression was investigated in the colony that survived against 5-Gy irradiation. The number of colonies made of CD133-positive cells apparently increased after 5-Gy irradiation to HepG2 (Figure 1d). This result indicated that CD133-negative cells are more sensitive to radiation than CD133-positive cells.
In order to characterize 82FR-31NR cells, we investigated mRNA expression of CD133, ABC transporters such as MDR1 (multi drug resistance 1) and BCRP1 (breast cancer resistance protein), albumin and GFAP (glial fibrillary acidic protein) by reverse transcription PCR in parental 0FR cells and 82FR-31NR cells (Figure 1e). As expected, CSC-related genes such as CD133 and BCRP1 were upregulated in 82FR-31NR cells compared with their parental cells in both cell lines. MDR1 expression is also higher in A172 82FR-31NR cells than that in A172 0FR cells. Conversely, the astrocyte marker GFAP was downregulated in A172 82FR-31NR cells compared with A172 0FR cells.
We further examined protein expression of liver stem cell marker cytokeratin14 in HepG2 cells by immunostaining. In contrast to parental 0FR cells, cytokeratin14 positive cells were observed in 82FR-31NR HepG2 (Figure 1f).
Using xenotransplntation into nude mice, we next confirmed tumorigenic potential of parental 0FR cells and 82FR-31NR cells from HepG2 and A172. Tumorigenic incidence was higher in 82FR-31NR cells than in parental 0FR cells (Table 1). These results demonstrated that exposure to FR for 82 days enriched CSCs owing to their radioresistance compared with non-stem cells.
Radioresistance of 82FR-31NR cells
We analyzed colony survival of 82FR-31NR cells after 2, 5 and 10-Gy irradiation. Compared with corresponding parental 0FR cells, significant radioresistance was observed at all the doses in 82 FR-31NR cells of both cell lines (Figure 2a). Fraction of annexin V positive apoptoic cells increased after 5-Gy irradiation in parental 0FR cells, whereas apoptosis was less induced in 82FR-31NR cells in both cell lines (Figure 2b).
Radioresistance of 82FR-31NR cells. (a) Survival curves of 0FR (open circle) and 82FR-31NR cells (closed circle) of HepG2 on the left and those of A172 on the right, after irradiation. Asterisks indicate significant radioresistance in 82FR-31NR cells as compared with 0FR cells. (b) Annexin V staining in 0FR and 31FR-31NR cells. Asterisks indicate significant increase of apoptotic cells by irradiation in 0FR and 82FR-31NR cells in comparison with non-irradiated cells. (c) The growth recovery after irradiation. Percentage of BrdU-positive cells was shown in right graph. FACS analysis was done on cells 25 h after 5-Gy irradiation. Asterisks indicate significant increase of BrdU-positive cells in 82FR-31NR cells with 5 Gy in comparison with 0FR cells with 5-Gy irradiation.
In order to analyze the growth recovery after radiation exposure, cells were irradiated with 5-Gy X-rays and were cultured for 24 h, pulse-labeled with BrdU (bromodeoxyuridine) for 1 h, and subjected to FACS analysis (Figure 2c). Exposure to 5 Gy decreased BrdU-positive cells in parental 0FR cells and 82FR-31NR cells in both cell lines, but the percentage of BrdU-positive cells was higher in 82FR-31NR cells than in parental 0FR cells 25 h after 5-Gy irradiation. This result showed that 82FR-31NR cells more proliferated than parental 0FR cells after irradiation.
Radiation-induced activation of the AKT/cyclin D1 pathway for efficient DNA repair in 82FR-31NR cells
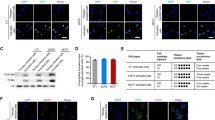
We studied DDR of 82FR-31NR cells to elucidate the molecular mechanisms underlying radioresistance of CSCs. We examined kinase activity of AKT in HepG2 parental 0FR cells and HepG2 82FR-31NR cells by determining phosphorylation at Serine473 (P-AKT Ser473) after irradiation (Figure 3a). Exposure to 5-Gy X-rays significantly induced apoptosis in parental 0FR cells, but not in 82FR-31NR cells (Figure 2b). AKT phosphorylation was observed 3 h after 5-Gy irradiation in 82FR-31NR cells and maintained for 24 h. However, AKT phosphorylation in parental 0FR cells was not increased at any time point examined after irradiation. The total AKT level in 82FR-31NR cells once decreased and then restored to the un-irradiated control level at 12 and 24 h after treatment, whereas the level tended to decrease in 0FR cells after irradiation. Cyclin D1, downstream of AKT pathway, was also upregulated following irradiation in 82FR-31NR cells with increased P-AKT Ser473 (Figure 3a). Conversely, cyclin D1 was degraded after DNA damage in parental 0FR cells.
Activation of the AKT/cyclin D1 pathway for efficient DNA repair in 82FR-31NR cells. (a) Western blotting of phosphorylated-AKT-Serine 473 (P-AKT Ser473), AKT, p53, cyclin D1 and β-actin in 0FR and 82FR-31NR cells. (b) Western blotting of γ-H2AX and β-actin in 0FR and 82FR-31NR cells of HepG2 and A172 after 5-Gy irradiation at indicated time points. (c) Western blotting of γ-H2AX and β-actin in 0FR and 82FR-31NR cells of HepG2 and A172 with radiation plus API-2. Cells were treated with 20 μM of API-2 for 24 h and then the drug was removed by washing out before irradiation. (d) Western blotting of γ-H2AX and β-actin in 0FR and 82FR-31NR cells of HepG2 and A172 by control siRNA (siControl) and cyclin D1 siRNA (siCD1) was shown. The amounts of γ-H2AX were normalized by corresponding β-actin level. The values are expressed relative to the untreated control value.
We speculated that induction of apoptosis by 5-Gy irradiation in parental 0FR cells was caused by activation of the p53 pathway, which regulates pro-apoptotic genes. As expected, the expression of p53 increased by irradiation in parental 0FR cells, whereas this was not observed in 82FR-31NR cells (Figure 3a). Interestingly, p53 was completely negative in 82FR-31NR cells regardless of exposure to acute radiation. Loss of p53 may associate with enrichment of CSCs induced by long-term FR.
We further examined correlation between AKT activation and DNA repair in 82FR-31NR cells. The repair kinetics of double strand breaks was examined by western blotting of γ-H2AX.19, 20 γ-H2AX was first detected 30 min after exposure to 5-Gy X-rays in 0FR and 82FR-31NR cells. The γ-H2AX level was restored to untreated control level (1.0) at 6 h after irradiation in 82FR-31NR cells, whereas the level still indicated >1.0 at that time in parental 0FR cells of both cell lines (Figure 3b). This efficient DNA repair in 82FR-31NR cells represented the nature of CSC. Upon treatment with API-2 or cyclin D1 siRNA, disappearance of γ-H2AX signal was suppressed and the γ-H2AX level was >1.0 at 6 h after irradiation in 82FR-31NR cells of both cell lines (Figures 3c and d). Thus, AKT/cyclin D1 pathway was associated with efficient DNA repair in 82FR-31NR cells after radiation.
Suppression of radioresistance in 82FR-31NR cells by inhibiting the AKT/cyclin D1 pathway
We studied the role of the AKT pathway in radioresistance of 82FR-31NR cells. We examined whether inhibiting the AKT/cyclin D1 pathway suppressed radioresistance of 82FR-31NR cells or not. We used API-2, rapamycin and a Cdk4 inhibitor (Cdk4-I), which suppresses the activity of cyclin D1/Cdk4. API-2 attenuated phosphorylation of AKT at Ser473 in 0FR and 82FR-31NR cells of HepG2. Rapamycin attenuated phosphorylation of downstream targets of mTOR including p70S6K and S6 in 0FR and 82FR-31NR cells of HepG2 (Figure 4a). We previously reported that 1.9 μM of Cdk4-I can suppress the kinase activity of cyclin D1/Cdk4 in HepG2 cells.16
Targeting the AKT/cyclin D1/Cdk4 pathway for suppression of radioresistance of 82FR-31NR cells. (a) Western blotting of CD133, P-AKT Ser473, AKT, phosphorylated-p70S6K-Threonine389 (P-P70S6K-Thr389), P70S6K, phosphorylated-S6-Serine240/Serine244 (P-S6-Ser240/244), S6 and β-actin in 0FR and 82FR-31NR cells with rapamycin or API-2. (b) Survival curves of 82FR-31NR cells (open circle), 82FR-31NR cells with API-2 (closed square), 82FR-31NR cells with Cdk4-I (closed triangle), 82FR-31NR cells with rapamycin (closed diamond) of HepG2 on the left and those of A172 on the right, after irradiation. Asterisks indicate significant radioresistance in 82FR-31NR cells with each drug compared with 82FR-31NR cells without drug. (c) Annexin V staining in 82FR-31NR cells. Asterisks indicate significant increase of Annexin V positive cells by treatment with drugs in 82FR-31NR cells in comparison with non-treated cells. (d) Cell growth in 82FR-31NR cells with 2 Gy of FR (closed circle), 82FR-31NR with FR plus API-2 (closed square), non-irradiated 82FR-31NR cells (open circle) and 82FR-31NR with API-2 (open square). Cells were treated with 20 μM of API-2 for 24 h once a week. (e) Cell growth in 82FR-31NR cells with 2 Gy of FR (closed circle), 82FR-31NR with FR plus Cdk4-I (closed triangle), non-irradiated 82FR-31NR cells (open circle) and 82FR-31NR with Cdk4-I (open triangle). Cells were treated with 1.9 μM of the Cdk4-I for 24 h once a week.
Treatment with API-2 or Cdk4-I rendered 82FR-31NR cells of HepG2 and A172 susceptibility to radiation, whereas rapamycin had no effect on the radiosensitivity of those cells (Figure 4b). Apoptosis was examined by annexin V staining in 82FR-31NR cells of HepG2 and A172 (Figure 4c). Annexin V positive apoptosis was induced by treatment with API-2 or Cdk4-I in 82FR-31NR cells, except in A172 83FR-31NR with rapamycin. Incidence of apoptosis was further increased by the combination of radiation and API-2 or Cdk4-I in 82FR-31NR cells in both cell lines, whereas the incidence of apoptosis was not affected by combination of radiation and rapamycin. These results demonstrated that the AKT/cyclin D1/Cdk4 pathway, but not the AKT/mTOR pathway, is responsible for radioresistance of 82FR-31NR cells.
In standard fractionated RT, multiple 2 Gy of FR was administrated to tumors. We investigated radiosensitivity to 2 Gy of FR in 82FR-31NR cells of HepG2 and A172 (Figures 4d and e). The growth of parental HepG2 and A172 cells was completely inhibited by 2 Gy of FR for 11 days.17 Although the growth was retarded by FR in 82FR-31NR cells of HepG2 and A172, they can grow during exposure of 2 Gy of FR. This radioresistance of 82FR-31NR cells against 2 Gy of FR was suppressed by the combination with either API-2 or Cdk4-I (Figures 4d and e). Therefore, we assumed that the AKT, cyclin D1 and Cdk4 are feasible molecular targets for suppression of radioresistance of CSCs.
We further assessed the importance of cyclin D1 and Cdk4 in radioresistance of 82FR-31NR cells. The expression of these proteins was suppressed by cyclin D1 siRNA and Cdk4 siRNA, respectively (Figure 5a). Radioresistance of 82FR-31NR cells disappeared by downregulation of cyclin D1 or Cdk4 concomitant with increased radiation-induced apoptosis (Figures 5a and b). These results demonstrated that cyclin D1 and Cdk4 are important molecular targets for the suppression of radioresistance in CSCs.
Suppression of radioresistance in 82FR-31NR cells by targeting cyclin D1/Cdk4. (a) Western blotting of cyclin D1, Cdk4 and β-actin by control siRNA (siControl), cyclin D1 siRNA (siCD1) and Cdk4 siRNA (siCdk4) are shown in the left panel. Western blotting was performed by using cell extracts collected 48 h after transfection. Survival curves of 82FR-31NR cells with siControl (open circle), siCD1 (closed triangle) or siCdk4 (closed square) after irradiation are shown. Cells were irradiated 24 h after transfection. Asterisks indicate significant radiosensitization in 82FR-31NR cells by siCD1 or siCdk4 compared with 82FR-31NR cells with siControl. (b) FACS analyses of apoptosis after 5-Gy irradiation on 82FR-31NR cells with siControl, siCD1 or siCdk4. Cells were irradiated 48 h after transfection and stained with Annexin V and propidium iodide. The percentages of apoptotic cells are shown on the right graph. Asterisk indicates that siCD1 or siCdk4 significantly induced apoptosis in 82FR-31NR cells compared with siControl.
Discussion
Enrichment of CSCs by radiation exposure
Tumor control is commonly assessed by reduction of the main tumor mass for evaluation of the efficacy of cancer treatment. The drawback of this estimation is that tumor regression by cancer treatment dose not correlate well with the loss of tumorigenicity. One surviving CSC has potential to make recurrent tumors after treatments. Therefore, it is tremendously important to eradicate CSCs completely for the cure of cancer.7 Most CSC studies have utilized a magnetic-beads-conjugated anti-CD133 antibody to isolate CSCs in a heterogeneous population.4 Other than this procedure, radiation and anti-cancer drug enrich radio- and chemo-resistant CSCs, which are implicated in treatment failures of RT or chemotherapy.21, 22, 23, 24
In this study, we have isolated surviving CSCs against FR by exposure to relatively low-dose X-rays for 82 days. We consider that our established 82FR-31NR cells are CSCs because they showed higher expression of CD133 and ABC transporters, high tumorigenic potential, radioresistance, and efficient DNA repair of radiation-induced DNA damage compared with their parental cell lines. CSCs were selected after exposure to FR for over two months and cultured in vitro for a long time, and the possibility remains that they are different from CSCs directly isolated from primary tumors. However, CSCs in the present study fulfilled the characteristics as CSCs. Isolation of CSC fraction by radiation was due to the selection of radioresistant CSCs or mutations toward the acquisition of the ability to self-renew in non-stem cells. Our data of preferential survival of CD133-positive cells against radiation damage indicated that radiation has an ability to enrich CSCs by selectively killing radiosensitive non-stem cells in an in vitro heterogeneous population.
Activation of the AKT/cyclin D1 pathway by radiation exposure in 82FR-31NR cells
Several internal cell signaling pathways, including the PI3K/AKT pathway, are activated by the serum-free culture condition specific to CSCs containing epidermal growth factor and basic fibroblast growth factor. These activations are not seen in the growth medium of monolayer cells.8 Both growth factors have previously been shown to decrease cellular radiosensitivity.25, 26 Therefore, it would be better to compare cells and their derived CSCs under the same culture condition for seeking for molecular targets of CSC treatments. Our isolated 82FR-31NR cells have an ability to self-renew even in the medium containing serum. Thus, we can analyze DDR of CSCs in as the same growth medium as that of parental cells
According to FACS analysis and western blotting data of 82FR-31NR cells, those cells can grow after irradiation with the activation of the AKT/cyclin D1 survival signaling pathway. In contrast, parental cells died after the same dose of irradiation without activation of the AKT pathway.
DDR regarding the AKT/cyclin D1 pathway in response to radiation is depicted in Figure 6. In parental cells, radiation-induced DNA damage activated p53, which promotes apoptosis in irradiated cells. In contrast, the AKT/cyclin D1/Cdk4 pathway was activated in 82FR-31NR cells by irradiation. Radiation-induced DNA damage was efficiently repaired in 82FR-31NR cells. Thus, the AKT/cyclin D1 pathway is a key to facilitate DNA repair of 82FR-31NR cells after irradiation. As reported previously, the PI3K/AKT pathway is implicated in DNA repair of radiation-induced DNA damage in glioma cells.27 Cyclin D1 regulates not only cell cycling, but also enhances DDR of homologous recombination repair (HRR) in cooperation with Rad51.28 We have previously reported that cyclin D1 knockdown by its siRNA decreases homologous recombination repair activity in radioresistant cells.16 Collectively, these observations indicate that cyclin D1 is a potent target to increase radiosensitivity of radioresistant cells by inhibiting homologous recombination repair. Indeed, DNA repair was impeded in 82FR-31NR cells by inhibiting the AKT/cyclin D1 pathway with API-2 or cyclin D1 siRNA. Thus, targeting the AKT pathway has the potential to induce cell death in CSCs by inhibiting efficient DNA repair, which is essential for protection of cells from irradiation.
DNA damage signaling pathway in parental cells and 82FR-31NR cells. Radiation activates the p53 pathway to induce apoptosis in irradiated parental cells. On the other hand, the AKT/cyclin D1/Cdk4 pathway is activated by irradiation in 82FR-31NR cells. The AKT pathway facilitates DNA repair of radiation-induced DNA damage and promotes cell survival in 82FR-31NR cells after irradiation.
AKT, cyclin D1and Cdk4 are molecular targets for eradication of CSC by RT
It is important to identify specific components of signal transduction pathway that is involved in radioresistance of CSCs. It was reported that the inhibition of the AKT pathway prevents neurosphere formation of glioblastoma cells and decreases cell growth of CD133-positive cells compared with CD133-negative cells.29 Inhibition of the PI3K/AKT pathway sensitizes CSCs to both radiation and anti-cancer drugs with induction of apoptosis in vivo.30, 31 The PI3K/AKT pathway promotes chemo-resistance by regulating the ABC transporter BCRP1 activity, which enhances drug efflux.32, 33, 34 In the present study, we have further demonstrated that cyclin D1/Cdk4 is the most important target downstream AKT to suppress radioresistance of CSCs. Thus, manipulation of cyclin D1 and Cdk4 may be useful to enhance radio- and chemo-sensitivity in CSCs. A number of therapeutic agents have been shown to induce cyclin D1 degradation.35 Cdk4 inhibitor is also considerable for treatment of mantle cell lymphoma and is now under phase 1 study.36, 37 In the future study, careful evaluation is required to confirm the efficacy of these cyclinD1/Cdk4-targeting drugs combined with fractionated RT.
We have reported that the constitutively activated AKT/cyclin D1 pathway is associated with acquired radioresistance of tumor cells induced by exposure to FR for 31 days.16 Because of CD133-negative in 31FR cells, cells with acquired radioresistance are different from CSCs. Furthermore, cells with acquired radioresistance harbor constitutive activation of the AKT/cyclin D1 pathway mediated by the cyclin D1 overexpression cycle, whereas AKT activation and cyclin D1 overexpression were not observed in non-irradiated 82FR-31NR cells as well as in non-irradiated parental cells. Our present data demonstrated that the AKT pathway is important not only in acquisition of radioresistance but also in radioresistance of CSCs. Thus, the AKT, cyclin D1 and Cdk4 are commonly implicated in intrinsic radioresistance of CSCs and acquired radioresistance by FR. Therefore, those molecules are attractive as therapeutic targets to overcome tumor radioresistance.
In conclusion, we have demonstrated that the AKT/cyclin D1/Cdk4 pathway may serve as a new target to enhance efficacy of RT by suppressing radioresistance of CSCs.
Materials and methods
Cell culture condition and drugs
HepG2, a human liver cancer cell line was obtained from the Cell Resource Center for Biomedical Research, IDAC, Tohoku University. A human glioblastoma cell line A172 was purchased from Japan Collection of Research Biosciences (Tokyo, Japan). Cells were grown in a RPMI1640 medium (NacalaiTesque, Kyoto, Japan) supplemented with 5% heat-inactivated fetal calf serum. An AKT inhibitor, API-2, and a Cdk4 inhibitor, Cdk4-I, were purchased from Calbiochem (San Diego, CA, USA). Rapamycin was purchased from Sigma (St Louis, MO, USA).
Irradiation experiments
Irradiation was performed using a 150-KVp X-ray generator (Model MBR-1520R, Hitachi, Tokyo, Japan) with a 0.5 mm Cu and 0.1 mm Al filter at a dose rate of 1.0 Gy per min.
Western blot analyses
Western blotting was performed as described previously.38 Proteins were separated by sodium-lauryl-sulfate-polyacrylamide gel electrophoresis and transferred electrophoretically to PVDF membranes (Bio-Rad, Richmond, CA, USA). Membranes were blocked with 5% (w/v) phospho-blocker (Cell Biolabs. Inc., San Diego, CA, USA) for 1 h and incubated with each primary antibody, such as anti-β-actin (A2066, Sigma), anti-phospho-AKT-Ser473 (4060, Cell Signaling, Beverly, MA, USA), anti-AKT (4685, Cell Signaling), anti-CD133 (3663, Cell Signaling), anti-cyclin D1 (Nichirei Bioscience, Tokyo, Japan), anti-γ-H2AX (Upstate, Lake Placid, NY, USA), anti-p53 (DAKO, Glostrup, Denmark), anti-phospho-S6-Ser240/244 (2215, Cell Signaling), anti-S6 (2211, Cell Signaling), anti-phospho-P70S6K-Thr389 (9205, Cell Signaling) and anti-P70S6K (9209, Cell Signaling) for 1 h at room temperature or overnight at 4 °C. Membranes were then incubated for 1 h at room temperature with the secondary antibody of HRP-conjugated goat anti-rabbit IgG (Nichirei Bioscience) or HRP-conjugated goat anti-mouse IgG (R&D Systems, Minneapolis, MN, USA). The protein bands were visualized with Chemi-Lumi One L western blotting substrate (NacalaiTesque). Band intensity was measured by densitometry using Multi Gauge Analyses software (Fuji Film, Tokyo, Japan).
Flow cytometry analysis of CD133 expression
Cells were stained with phycoerythrin-conjugated anti-CD133 antibody (MilyenyiBiotec Inc., Auburn, CA, USA) following the manufacturer’s protocol. Samples were analyzed on the FACScan (Cytomics FC500, Becton Dickinson, Mountain View, CA, USA).
Reverse transcription PCR
The expression of each gene was examined by RT (reverse transcription)–PCR. The primer sets used in the present analysis were as follows. Albumin: 5′-CGGCTTATTCCAGGGGTGTG-3′ and 5′-GGGGGAGGTTTGGGTTGTC-3′; BCRP1: 5'-AGATGGGTTTCCAAGCGTTCAT-3' and 5'-CCAGTCCCAGTACGACTGTGACA -3'; CD133: 5'-CATAAAGCTGGACCCATTGG-3' and 5'-CCTTGTCCTTGGTAGTGTTG-3′; GADPH: 5'-GCCAAAAGGGTCATCATCTC-3' and 5'-GTAGAGGCAGGGATGATGTTC -3'; GFAP: 5′-GCTCGATCAACTCACCGCCAACA-3′ and 5′-GGGCAGCAGCGTCTGTCAGGTC-3′; MDR1: 5′-ATATCAGCAGCCCACATCAT-3′ and 5′-GAAGCACTGGGATGTCCGGT -3′;
The PCR products were separated by electrophoresis in 1% agarose gel.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously.39 Cells were grown on 18 mm × 18 mm cover slips. Cells were fixed in 4% (w/v) paraformaldehyde in phosphate buffered saline (PBS) for 10 min, washed in PBS, made permeable in 0.25% Triton X-100 for 10 min, and then washed and blocked with PBS containing 5% bovine serum albumin for 15 min (permeabilization was omitted for CD133 staining). Cells were incubated with anti-CD133 (130-090-851, MilyenyiBiotec Inc.) or anti-cytokeratin14 (MAB3232, Millipore) antibodies. Antibodies were diluted in PBS with 0.5% bovine serum albumin for 1 h at 37 °C. Slides were then washed three times with 0.1% Triton X-100 in PBS and incubated for 1 h at 37 °C with secondary antibody conjugated with Alexa 488 (Molecular Probes, Eugene, OR, USA) or Cy-3 (Jackson Immuno Research Laboratories, Westgrove, PA, USA). Slides were washed three times with 0.1% Triton X-100 in PBS and counterstained for DNA with Hochest33258 (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Images were captured by an epifluorescense microscopy (Nikon, Tokyo, Japan) using a × 40 objective lens.
Clonogenic assay
Cells were seeded in 60-mm dish coated with 0.1% gelatin (Wako Pure Chemical Industries, Ltd) at 1 × 103 cells per dish. After irradiation, cells were incubated for 10 days until colonies were visible. They were fixed with ethanol for 30 min and stained with Giemsa solution (Merck & Co. Inc. Pennsylvania, PA, USA). The colonies of >50 cells were counted under a light microscope.
Annexin V staining
Apoptotic cells were identified and quantified using an annexin V-FITC apoptosis detection kit (Bio Vision, Mountain View, CA, USA) following the manufacturer’s protocol. Cells were stained with annexin V-FITC and propidium iodide 48 h after treatment with radiation. Annexin V positive apoptotic cells were analyzed by the FACScan (Becton Dickinson).
RNA interference
Cells were transfected with siRNA using a Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA). Cyclin D1 siRNA, Cdk4 siRNA and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animal experiments
The study design was approved by the Ethical Committee of IDAC, Tohoku University. Male BALB/c nu/nu mice of 5 weeks of age were used in the present experiment. All mice were maintained in our animal facility on a 12 light and 12 dark hour cycle under a controlled temperature (23±2 °C). For the transplantation, 5 × 105 cells of 0FR and 82FR-31NR of HepG2 and A172 in 100 μl of saline were injected into the right and the left legs of the mice, respectively.
Cell growth assay
Cells (5 × 105) were seeded in a 25 cm2 flask (Thermo Fisher Scientific/Nunc, Waltham, MA, USA), incubated overnight and irradiated daily with 2 Gy of FR for the indicated number of days. Growth rates were monitored by counting cell number twice a week. When the total cell number was over 5 × 105, cells were sub-cultured to 5 × 105 cells in a flask.
Statistical analysis
Error bars represent standard deviations; in some cases the standard deviations were too small to be visible on the histogram. All the experiments were repeated at least three times with independent samples. Student’s t-test was used for the analysis. A single asterisk and double asterisks indicate the significance, (P<0.01) and (P<0.05), respectively.
References
Kim JJ, Tannock IF . Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 2005; 5: 516–525.
Pardal R, Clarke MF, Morrison SJ . Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 2003; 3: 895–902.
Nguyen GH, Murph MM, Chang JY . Cancer stem cell radioresistance and enrichment: where frontline radiation therapy may fail in lung and esophageal cancers. Cancers 2011; 3: 1232–1252.
Bao SD, Wu QL, McLendon RE, Hao YL, Shi Q, Hjelmeland AB et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760.
Baumann M, Krause M, Hill R . Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 2008; 8: 545–554.
Phillips TM, McBride WH, Pajonk F . The response of CD24(−/low)/CD44(+) breast cancer-initiating cells to radiation. JNatl Cancer Inst 2006; 98: 1777–1785.
Tu LC, Foltz G, Lin E, Hood L, Tian Q . Targeting stem cells-clinical implications for cancer therapy. Curr Stem Cell Res Ther 2009; 4: 147–153.
Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA 2009; 106: 268–273.
Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-Catenin signaling. Plos Biology 2009; 7: e1000121.
Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M . Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 2003; 102: 972–980.
Schmidt-Ullrich RK, Contessa JN, Dent P, Mikkelsen RB, Valerie K, Reardon DB et al. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig 1999; 7: 321–330.
Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res 2003; 159: 283–300.
Manning BD, Cantley LC . AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274.
Vivanco I, Sawyers CL . The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL . Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011; 11: 558–572.
Shimura T, Kakuda S, Ochiai Y, Nakagawa H, Kuwahara Y, Takai Y et al. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3 beta-mediated cyclin D1 overexpression. Oncogene 2010; 29: 4826–4837.
Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M . Targeting the AKT/GSK3 beta/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. Int J Radiat Oncol Biol Phys 2011; 80: 540–548.
Wu YJ, Wu PY . CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev 2009; 18: 1127–1134.
Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM . Characteristics of gamma-H2AX foci at DNA double strand breaks sites. Biochem Cell Biol 2003; 81: 123–129.
Sedelnikova OA, Pilch DR, Redon C, Bonner WM . Histone H2AX in DNA damage and repair. Cancer Biol Ther 2003; 2: 233–235.
Ma L, Lai DM, Liu T, Cheng WW, Guo LH . Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin 2010; 42: 593–602.
Mihatsch J, Toulany M, Bareiss PM, Grimm S, Lengerke C, Kehlbach R et al. Selection of radioresistant tumor cells and presence of ALDH1 activity in vitro. Radiother Onco 2011; 99: 300–306.
Che SM, Liu XL, Chen X, Hou L, Zhang XZ . The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis Esophagus 2011; 24: 265–273.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1: 313–323.
Wollman R, Yahalom J, Maxy R, Pinto J, Fuks Z . Effect of epidermal growth-factor on the growth and radiation sensitivity of human breast-cancer cells in-vitro. Int J Radiat Oncol Biol Phys 1994; 30: 91–98.
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001; 293: 293–297.
Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A . Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem 2007; 282: 21206–21212.
Jirawatnotai S, Hu YD, Michowski W, Elias JE, Becks L, Bienvenu F et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature 2011; 474: 230–234.
Eyler CE, Foo WC, Lafiura KM, McLendon RE, Hjelmeland AB, Rich JN . Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 2008; 26: 3027–3036.
Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC . PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev 2008; 22: 436–448.
Ma S, Lee TK, Zheng BJ, Chan K, Guan XY . CD133(+) HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008; 27: 1749–1758.
Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 2009; 4: 226–235.
Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem 2003; 278: 39068–39075.
Takada T, Suzuki H, Gotoh Y, Sugiyama Y . Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos 2005; 33: 905–909.
Alao JP . The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007; 6: 24.
Korz C, Pscherer A, Benner A, Mertens D, Schaffner C, Leupolt E et al. Evidence for distinct pathomechanisms in B-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood 2002; 99: 4554–4561.
Marzec M, Kasprzycka M, Lai R, Gladden AB, Wlodarski P, Tomczak E et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 2006; 108: 1744–1750.
Shimura T, Toyoshima M, Adiga SK, Kunoh T, Nagai H, Shimizu N et al. Suppression of replication fork progression in low-dose-specic p53-dependent S-phase DNA damage checkpoint. Oncogene 2006; 25: 5921–5932.
Shimura T, Torres MJ, Martin MM, Rao VA, Pommier Y, Katsura M et al. Bloom's syndrome helicase and Mus81 are required to induce transient double-strand DNA breaks in response to DNA replication stress. J Mol Biol 2008; 375: 1152–1164.
Acknowledgements
This study was partly supported by the Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, for Cancer Research from the Ministry of Health, Labour and Welfare, Research Seeds Quest Program of JST, Radiation Effect Association and Mishima Kaiun Memorial Foundation of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shimura, T., Noma, N., Oikawa, T. et al. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis 1, e12 (2012). https://doi.org/10.1038/oncsis.2012.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2012.12
Keywords
This article is cited by
-
The reversibility of cancer radioresistance: a novel potential way to identify factors contributing to tumor radioresistance
Human Cell (2023)
-
Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice
Communications Biology (2019)
-
Repeated photon and C-ion irradiations in vivo have different impact on alteration of tumor characteristics
Scientific Reports (2018)
-
HCRP1 downregulation confers poor prognosis and induces chemoresistance through regulation of EGFR-AKT pathway in human gastric cancer
Virchows Archiv (2017)
-
CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-κB pathway in multidrug-resistant glioblastoma cells in vitro
Oncogene (2016)