Abstract
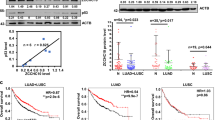
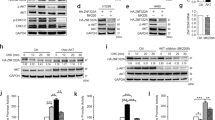
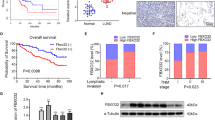
Overexpression of Cys2His2 zinc-finger 322A (ZNF322A) oncogenic transcription factor is associated with lung tumorigenesis. However, the mechanism of ZNF322A overexpression remains poorly understood. Here, we discover that protein stability of ZNF322A is regulated by coordinated phosphorylation and ubiquitination through the CK1δ/GSK3β/FBXW7α axis. CK1δ and GSK3β kinases sequentially phosphorylate ZNF322A at serine-396 and then serine-391. Moreover, the doubly phosphorylated ZNF322A protein creates a destruction motif for the ubiquitin ligase FBXW7α leading to ZNF322A protein destruction. Overexpression of FBXW7α induces ZNF322A protein degradation, thereby blocks ZNF322A transcription activity and suppresses ZNF322A-induced tumor growth and metastasis in vitro and in vivo. Clinically, overexpression of ZNF322A correlates with low FBXW7α or defective CK1δ/GSK3β-mediated phosphorylation in lung cancer patients. Multivariate Cox regression analysis indicates that patients with ZNF322A high/FBXW7 low expression profile can be used as an independent factor to predict the clinical outcome in lung cancer patients. Our results reveal a new mechanism of ZNF322A oncoprotein destruction regulated by the CK1δ/GSK3β/FBXW7α axis. Deregulation of this signaling axis results in ZNF322A overexpression and promotes cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wolfe SA, Nekludova L, Pabo CO . DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct 2000; 29: 183–212.
Jia D, Hasso SM, Chan J, Filingeri D, D'Amore PA, Rice L et al. Transcriptional repression of VEGF by ZNF24: mechanistic studies and vascular consequences in vivo. Blood 2013; 121: 707–715.
Yu J, Liang QY, Wang J, Cheng Y, Wang S, Poon TC et al. Zinc-finger protein 331, a novel putative tumor suppressor, suppresses growth and invasiveness of gastric cancer. Oncogene 2013; 32: 307–317.
Thollet A, Vendrell JA, Payen L, Ghayad SE, Ben Larbi S, Grisard E et al. ZNF217 confers resistance to the pro-apoptotic signals of paclitaxel and aberrant expression of Aurora-A in breast cancer cells. Mol Cancer 2010; 9: 291.
Vendrell JA, Thollet A, Nguyen NT, Ghayad SE, Vinot S, Bièche I et al. ZNF217 is a marker of poor prognosis in breast cancer that drives epithelial-mesenchymal transition and invasion. Cancer Res 2012; 72: 3593–3606.
Jen J, Lin LL, Chen HT, Liao SY, Lo FY, Tang YA et al. Oncoprotein ZNF322A transcriptionally deregulates alpha-adducin, cyclin D1 and p53 to promote tumor growth and metastasis in lung cancer. Oncogene 2016; 35: 2357–2369.
Kao SH, Wang WL, Chen CY, Chang YL, Wu YY, Wang YT et al. GSK3beta controls epithelial-mesenchymal transition and tumor metastasis by CHIP-mediated degradation of Slug. Oncogene 2014; 33: 3172–3182.
Wang R, Wang Y, Liu N, Ren C, Jiang C, Zhang K et al. FBW7 regulates endothelial functions by targeting KLF2 for ubiquitination and degradation. Cell Res 2013; 23: 803–819.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6: 931–940.
Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park SH et al. Role of CK1 in GSK3beta-mediated phosphorylation and degradation of snail. Oncogene 2010; 29: 3124–3133.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002; 108: 837–847.
Hergovich A, Lisztwan J, Thoma CR, Wirbelauer C, Barry RE, Krek W . Priming-dependent phosphorylation and regulation of the tumor suppressor pVHL by glycogen synthase kinase 3. Mol Cell Biol 2006; 26: 5784–5796.
Welcker M, Clurman BE . FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008; 8: 83–93.
Wang Z, Liu P, Inuzuka H, Wei W . Roles of F-box proteins in cancer. Nat Rev Cancer 2014; 14: 233–247.
Busino L, Millman SE, Scotto L, Kyratsous CA, Basrur V, O'Connor O et al. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat Cell Biol 2012; 14: 375–385.
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011; 471: 104–109.
Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG Jr. . The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 2005; 8: 25–33.
Zhao D, Zheng HQ, Zhou Z, Chen C . The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res 2010; 70: 4728–4738.
Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP . Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell 2007; 26: 131–143.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116: 1175–1186.
Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M et al. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA 2003; 100: 10193–10200.
Skaar JR, Pagan JK, Pagano M . Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol 2013; 14: 369–381.
Davis RJ, Welcker M, Clurman B . Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 2014; 26: 455–464.
Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011; 471: 110–114.
Gan W, Dai X, Lunardi A, Li Z, Inuzuka H, Liu P et al. SPOP promotes ubiquitination and degradation of the ERG oncoprotein to suppress prostate cancer progression. Mol Cell 2015; 59: 917–930.
Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S et al. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 2010; 18: 147–159.
Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung MC et al. GSK-3 beta targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell 2008; 13: 36–47.
Pontano LL, Aggarwal P, Barbash O, Brown EJ, Bassing CH, Diehl JA . Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol 2008; 28: 7245–7258.
Acknowledgements
We are grateful for the support from the Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital. We thank Mr Chien-Hsun Lin for providing technical support. We acknowledge Dr Ruey-Hwa Chen, Institute of Biological Chemistry, Academia Sinica for discussion and comments on the manuscript. This work was supported in part by the Taiwan Ministry of Science and Technology grants (104-2627-B-006-001; 104-2627-B-002-001) and the Aim for the Top University Project grant from the Taiwan Ministry of Education (D105-35A07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Liao, SY., Chiang, CW., Hsu, CH. et al. CK1δ/GSK3β/FBXW7α axis promotes degradation of the ZNF322A oncoprotein to suppress lung cancer progression. Oncogene 36, 5722–5733 (2017). https://doi.org/10.1038/onc.2017.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.168
This article is cited by
-
ZNF322A-mediated protein phosphorylation induces autophagosome formation through modulation of IRS1-AKT glucose uptake and HSP-elicited UPR in lung cancer
Journal of Biomedical Science (2020)
-
AKT-mediated phosphorylation enhances protein stability and transcription activity of ZNF322A to promote lung cancer progression
Oncogene (2019)
-
Identification of aberrantly expressed F-box proteins in squamous-cell lung carcinoma
Journal of Cancer Research and Clinical Oncology (2018)