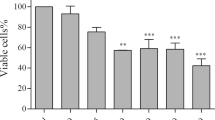
Abstract
Resveratrol (trans-3,5,4′-truhydroxystilbene) possesses a strong anticancer activity exhibited as the induction of apoptosis through p53 activation. However, the molecular mechanism and direct target(s) of resveratrol-induced p53 activation remain elusive. Here, the Ras-GTPase-activating protein SH3 domain-binding protein 1 (G3BP1) was identified as a potential target of resveratrol, and in vitro binding assay results using resveratrol-conjugated Sepharose 4B beads confirmed their direct binding. Depletion of G3BP1 significantly diminishes resveratrol-induced p53 expression and apoptosis. We also found that G3BP1 negatively regulates p53 expression by interacting with ubiquitin-specific protease 10 (USP10), a deubiquitinating enzyme of p53. Disruption of the interaction of p53 with USP10 by G3BP1 interference leads to the suppression of p53 deubiquitination. Resveratrol, on the other hand, directly binds to G3BP1 and prevents the G3BP1/USP10 interaction, resulting in enhanced USP10-mediated deubiquitination of p53, and consequently increased p53 expression. These findings disclose a novel mechanism of resveratrol-induced p53 activation and resveratrol-induced apoptosis by direct targeting of G3BP1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000; 408: 307–310.
Oren M . Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10: 431–442.
Celotti E, Ferrarini R, Zironi R, Conte LS . Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J Chromatogr A 1996; 730: 47–52.
Chen RS, Wu PL, Chiou RY . Peanut roots as a source of resveratrol. J Agric Food Chem 2002; 50: 1665–1667.
Sobolev VS, Cole RJ . Trans-resveratrol content in commercial peanuts and peanut products. J Agric Food Chem 1999; 47: 1435–1439.
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–220.
Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem 2003; 278: 41482–41490.
Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 2003; 102: 987–995.
Fulda S, Debatin KM . Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res 2004; 64: 337–346.
Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW . Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate 2007; 67: 1641–1653.
Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG . Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett 2003; 190: 157–163.
Pervaiz S . Resveratrol: from grapevines to mammalian biology. FASEB J 2003; 17: 1975–1985.
She QB, Bode AM, Ma WY, Chen NY, Dong Z . Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res 2001; 61: 1604–1610.
Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM . Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res 1999; 59: 2596–2601.
Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB . Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res 2002; 8: 893–903.
Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, Debussche L et al. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell Biol 1996; 16: 2561–2569.
Pazman C, Mayes CA, Fanto M, Haynes SR, Mlodzik M . Rasputin, the Drosophila homologue of the RasGAP SH3 binding protein, functions in ras- and Rho-mediated signaling. Development 2000; 127: 1715–1725.
Tourriere H, Gallouzi IE, Chebli K, Capony JP, Mouaikel J, van der Geer P et al. RasGAP-associated endoribonuclease G3Bp: selective RNA degradation and phosphorylation-dependent localization. Mol Cell Biol 2001; 21: 7747–7760.
Gallouzi IE, Parker F, Chebli K, Maurier F, Labourier E, Barlat I et al. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol Cell Biol 1998; 18: 3956–3965.
Prigent M, Barlat I, Langen H, Dargemont C . IkappaBalpha and IkappaBalpha/NF-kappa B complexes are retained in the cytoplasm through interaction with a novel partner, RasGAP SH3-binding protein 2. J Biol Chem 2000; 275: 36441–36449.
Barnes CJ, Li F, Mandal M, Yang Z, Sahin AA, Kumar R . Heregulin induces expression, ATPase activity, and nuclear localization of G3BP, a Ras signaling component, in human breast tumors. Cancer Res 2002; 62: 1251–1255.
Liu Y, Zheng J, Fang W, You J, Wang J, Cui X et al. Identification of metastasis associated gene G3BP by differential display in human cancer cell sublines with different metastatic potentials G3BP as highly expressed in non-metastatic. Chin Med J (Engl) 2001; 114: 35–38.
French J, Stirling R, Walsh M, Kennedy HD . The expression of Ras-GTPase activating protein SH3 domain-binding proteins, G3BPs, in human breast cancers. Histochem J 2002; 34: 223–231.
Gautier-Bert K, Murol B, Jarrousse AS, Ballut L, Badaoui S, Petit F et al. Substrate affinity and substrate specificity of proteasomes with RNase activity. Mol Biol Rep 2003; 30: 1–7.
Soncini C, Berdo I, Draetta G . Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene 2001; 20: 3869–3879.
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z . USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 2010; 140: 384–396.
Kim MM, Wiederschain D, Kennedy D, Hansen E, Yuan ZM . Modulation of p53 and MDM2 activity by novel interaction with Ras-GAP binding proteins (G3BP). Oncogene 2007; 26: 4209–4215.
Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M . Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 2013; 18: 135–146.
Takahashi M, Higuchi M, Matsuki H, Yoshita M, Ohsawa T, Oie M et al. Stress granules inhibit apoptosis by reducing reactive oxygen species production. Mol Cell Biol 2013; 33: 815–829.
Chow SE, Wang JS, Chuang SF, Chang YL, Chu WK, Chen WS et al. Resveratrol-induced p53-independent apoptosis of human nasopharyngeal carcinoma cells is correlated with the downregulation of DeltaNp63. Cancer Gene Ther 2010; 17: 872–882.
Zhang H, Zhang S, He H, Zhao W, Chen J, Shao RG . GAP161 targets and downregulates G3BP to suppress cell growth and potentiate cisplaitin-mediated cytotoxicity to colon carcinoma HCT116 cells. Cancer Sci 2012; 103: 1848–1856.
Annibaldi A, Dousse A, Martin S, Tazi J, Widmann C . Revisiting G3BP1 as a RasGAP binding protein: sensitization of tumor cells to chemotherapy by the RasGAP 317-326 sequence does not involve G3BP1. PLoS One 2011; 6: e29024.
Oi N, Jeong CH, Nadas J, Cho YY, Pugliese A, Bode AM et al. Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene A(4)hydrolase. Cancer Res 2010; 70: 9755–9764.
Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW et al. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinogen 2008; 47: 797–805.
Thangima Zannat M, Bhattacharjee RB, Bag J . In the absence of cellular poly (A) binding protein, the glycolytic enzyme GAPDH translocated to the cell nucleus and activated the GAPDH mediated apoptotic pathway by enhancing acetylation and serine 46 phosphorylation of p53. Biochem Biophys Res Commun 2011; 409: 171–176.
Gray-Schopfer V, Wellbrock C, Marais R . Melanoma biology and new targeted therapy. Nature 2007; 445: 851–857.
Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M . Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther 2003; 2: 753–763.
Castresana JS, Rubio MP, Vazquez JJ, Idoate M, Sober AJ, Seizinger BR et al. Lack of allelic deletion and point mutation as mechanisms of p53 activation in human malignant melanoma. Int J Cancer 1993; 55: 562–565.
Albino AP, Vidal MJ, McNutt NS, Shea CR, Prieto VG, Nanus DM et al. Mutation and expression of the p53 gene in human malignant melanoma. Melanoma Res 1994; 4: 35–45.
Hartmann A, Blaszyk H, Cunningham JS, McGovern RM, Schroeder JS, Helander SD et al. Overexpression and mutations of p53 in metastatic malignant melanomas. Int J Cancer 1996; 67: 313–317.
Sowa ME, Bennett EJ, Gygi SP, Harper JW . Defining the human deubiquitinating enzyme interaction landscape. Cell 2009; 138: 389–403.
Acknowledgements
We thank Dr Yibin Deng (The Hormel Institute, University of Minnesota, Austin, MN, USA) for kindly providing the expression vectors of shRNA p53. This work was supported by AgStar Minnesota (to ZD), The Hormel Foundation (to ZD) and National Institutes of Health Grants CA111536 (to ZD), CA172457 (to ZD), CA166011 (to ZD), R37 CA081064 (to ZD), CA148940 (to ZL), CA108961 (to ZL), the National Basic Research Program of China (973 Program, Grant No. 2013CB530700), National Natural Science Foundation of China (81102011, 81222029 and 31270806) and Kendall-Mayo Fellowship in Biochemistry, the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81221001) and Shanghai Science and Technology Committee Modernization of Traditional Chinese Medicine special (11DZ1973801).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Oi, N., Yuan, J., Malakhova, M. et al. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. Oncogene 34, 2660–2671 (2015). https://doi.org/10.1038/onc.2014.194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.194
This article is cited by
-
CircNUP50 is a novel therapeutic target that promotes cisplatin resistance in ovarian cancer by modulating p53 ubiquitination
Journal of Nanobiotechnology (2024)
-
G3BP1 interacts with YWHAZ to regulate chemoresistance and predict adjuvant chemotherapy benefit in gastric cancer
British Journal of Cancer (2021)
-
G3BP1 promotes tumor progression and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal cell carcinomas
Cell Death & Disease (2018)