Abstract
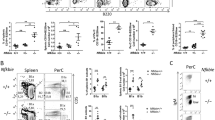
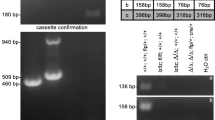
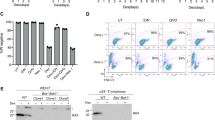
Proapoptotic Bcl-2 family members of the Bcl-2 homology (BH)3-only subgroup are critical for the establishment and maintenance of tissue homeostasis and can mediate apoptotic cell death in response to developmental cues or exogenously induced forms of cell stress. On the basis of the biochemical experiments as well as genetic studies in mice, the BH3-only proteins Bad and Bmf have been implicated in different proapoptotic events such as those triggered by glucose- or trophic factor-deprivation, glucocorticoids, or histone deacetylase inhibition, as well as suppression of B-cell lymphomagenesis upon aberrant expression of c-Myc. To address possible redundancies in cell death regulation and tumor suppression, we generated compound mutant mice lacking both genes. Our studies revealed lack of redundancy in most paradigms of lymphocyte apoptosis tested in tissue culture. Only spontaneous cell death of thymocytes kept in low glucose or that of pre-B cells deprived of cytokines was significantly delayed when both genes were lacking. Of note, despite these minor apoptosis defects we observed compromised lymphocyte homeostasis in vivo that affected mainly the B-cell lineage. Long-term follow-up revealed significantly reduced latency to spontaneous tumor formation in aged mice when both genes were lacking. Together our study suggests that Bad and Bmf co-regulate lymphocyte homeostasis and limit spontaneous transformation by mechanisms that may not exclusively be linked to the induction of lymphocyte apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Chipuk JE, Green DR . How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 2008; 18: 157–164.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ . Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183–192.
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17: 525–535.
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005; 17: 393–403.
Maiuri MC, Le TG, Criollo A, Rain JC, Gautier F, Juin P et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 2007; 26: 2527–2539.
Danial NN . BAD: undertaker by night, candyman by day. Oncogene 2008; 27 (Suppl 1): S53–S70.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008; 135: 1311–1323.
Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9324–9329.
Kelly PN, White MJ, Goschnick MW, Fairfax KA, Tarlinton DM, Kinkel SA et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ 2010; 17: 1655–1664.
Frenzel A, Labi V, Chmelewskij W, Ploner C, Geley S, Fiegl H et al. Suppression of B-cell lymphomagenesis by the BH3-only proteins Bmf and Bad. Blood 2010; 115: 995–1005.
Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y et al. Regulation of PTEN transcription by p53. Mol Cell 2001; 8: 317–325.
Vivanco I, Sawyers CL . The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501.
Engelman JA . Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009; 9: 550–562.
Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 2000; 6: 41–51.
Lee JW, Soung YH, Kim SY, Nam SW, Kim CJ, Cho YG et al. Inactivating mutations of proapoptotic Bad gene in human colon cancers. Carcinogenesis 2004; 25: 1371–1376.
Teo K, Gemmell L, Mukherjee R, Traynor P, Edwards J . Bad expression influences time to androgen escape in prostate cancer. BJU Int 2007; 100: 691–696.
Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA 2006; 103: 14907–14912.
Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O’Hare MJ et al. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem 2002; 277: 27643–27650.
Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003; 424: 952–956.
Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001; 293: 1829–1832.
Pinon JD, Labi V, Egle A, Villunger A . Bim and Bmf in tissue homeostasis and malignant disease. Oncogene 2008; 27 (Suppl 1): S41–S52.
Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med 2008; 205: 641–655.
Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia 2008; 22: 370–377.
Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738.
Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005; 106: 4131–4138.
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951.
Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A . Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol 2004; 24: 1570–1581.
Hubner A, Cavanagh-Kyros J, Rincon M, Flavell RA, Davis RJ . Functional cooperation of the proapoptotic Bcl2 family proteins Bmf and Bim in vivo. Mol Cell Biol 2010; 30: 98–105.
Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC . Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem 2008; 283: 36344–36353.
Wang P, Kang D, Cao W, Wang Y, Liu Z . Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev 2011; 28: 109–122.
Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA et al. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res 2009; 15: 5073–5081.
Villunger A, Huang DC, Holler N, Tschopp J, Strasser A . Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J Immunol 2000; 165: 1337–1343.
Acknowledgements
We thank A Strasser and the late S Korsemeyer for the mice and reagents. I Gaggl for mouse genotyping, C Soratroi for technical assistance, K Rossi for animal care and G Böck for cell sorting. This work was supported by grants from the ‘Tiroler Krebshilfe’ to FB and CW, the ‘Tiroler Wissenschaftsfond’ (TWF) to VL, as well as the Austrian Science Fund, FWF (Y212-B12; P23510-B19; SFB021) to AV and the Integrated Center for Research and Therapy (IFTZ) of the Innsbruck Medical University to AV and FB. CW is a recipient of a DOC-fFORTE doctoral fellowship sponsored by the Austrian Academy of Science (ÖAW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Baumgartner, F., Woess, C., Pedit, V. et al. Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene 32, 621–630 (2013). https://doi.org/10.1038/onc.2012.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.78
Keywords
This article is cited by
-
Combined loss of the BH3-only proteins Bim and Bmf restores B-cell development and function in TACI-Ig transgenic mice
Cell Death & Differentiation (2015)
-
Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death
Cell Death & Disease (2013)