Abstract
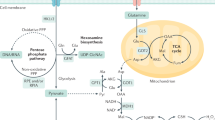
Tumor cells require increased adenosine triphosphate (ATP) to support anabolism and proliferation. The precise mechanisms regulating this process in tumor cells are unknown. Here, we show that the receptor for advanced glycation endproducts (RAGE) and one of its primary ligands, high-mobility group box 1 (HMGB1), are required for optimal mitochondrial function within tumors. We found that RAGE is present in the mitochondria of cultured tumor cells as well as primary tumors. RAGE and HMGB1 coordinately enhanced tumor cell mitochondrial complex I activity, ATP production, tumor cell proliferation and migration. Lack of RAGE or inhibition of HMGB1 release diminished ATP production and slowed tumor growth in vitro and in vivo. These findings link, for the first time, the HMGB1–RAGE pathway with changes in bioenergetics. Moreover, our observations provide a novel mechanism within the tumor microenvironment by which necrosis and inflammation promote tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Vakkila J, Lotze MT . Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004; 4: 641–648.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Tang D, Kang R, Zeh HJ, Lotze MT . High-mobility group box 1 and cancer. Biochim Biophys Acta 2010; 1799: 131–140.
Lotze MT, Tracey KJ . High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005; 5: 331–342.
Kang R, Tang D, Livesey KM, Schapiro NE, Lotze MT, Zeh HJ . The receptor for advanced glycation end-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid Redox Signal 2011; 15: 2175–2184.
Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 2010; 17: 666–676.
Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 1999; 22: 276–280.
Kang R, Louxa T, Tang D, Schapiroa NE, Vernona P, Liveseya KM et al. RAGE expression is permissive for early pancreatic neoplasia. Proc Natl Acad Sci USA 2012.
Scaffidi P, Misteli T, Bianchi ME . Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195.
Bianchi ME . HMGB1 loves company. J Leukoc Biol 2009; 86: 573–576.
Mantovani A . Cancer: inflaming metastasis. Nature 2009; 457: 36–37.
Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 2004; 164: 441–449.
Rai V, Maldonado AY, Burz DS, Reverdatto S, Schmidt AM, Shekhtman A . Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem 2012; 287: 5133–5144.
Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem 2008; 283: 34457–34468.
Zhang Y, Li W, Zhu S, Jundoria A, Li J, Yang H et al. Tanshinone IIA sodium sulfonate facilitates endocytic HMGB1 uptake. Biochem Pharmacol 2012; 84: 1492–1500.
Schmidt AM, Yan SD, Yan SF, Stern DM . The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001; 108: 949–955.
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J et al. RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 2009; 7: 17.
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T et al. Function of mitochondrial Stat3 in cellular respiration. Science 2009; 323: 793–797.
Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab 2011; 13: 701–711.
Pagliarini DJ, Dixon JE . Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci 2006; 31: 26–34.
Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 2006; 176: 12–15.
Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X . The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol 2007; 179: 1236–1244.
Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA 2002; 99: 12351–12356.
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res 2012; 72: 230–238.
Livesey K, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res 2012; 72: 1996–2005.
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P et al. Endogenous HMGB1 regulates autophagy. J Cell Biol 2010; 190: 881–892.
Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010; 29: 5299–5310.
Andersson U, Tracey KJ . HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011; 29: 139–162.
Stumbo AC, Cortez E, Rodrigues CA, Henriques MG, Porto LC, Barbosa HS et al. Mitochondrial localization of non-histone protein HMGB1 during human endothelial cell-Toxoplasma gondii infection. Cell Biol Int 2008; 32: 235–238.
Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S et al. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS ONE 2008; 3: e1443.
Alonso M, Melani M, Converso D, Jaitovich A, Paz C, Carreras MC et al. Mitochondrial extracellular signal-regulated kinases 1/2 (ERK1/2) are modulated during brain development. J Neurochem 2004; 89: 248–256.
Antico Arciuch VG, Alippe Y, Carreras MC, Poderoso JJ . Mitochondrial kinases in cell signaling: facts and perspectives. Adv Drug Deliv Rev 2009; 61: 1234–1249.
Galli S, Jahn O, Hitt R, Hesse D, Opitz L, Plessmann U et al. A new paradigm for MAPK: structural interactions of hERK1 with mitochondria in HeLa cells. PLoS ONE 2009; 4: e7541.
Gefter JV, Shaufl AL, Fink MP . Delude RL. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res 2009; 337: 79–89.
Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 2008; 205: 275–285.
DiNorcia J, Lee MK, Moroziewicz DN, Winner M, Suman P, Bao F et al. RAGE gene deletion inhibits the development and progression of ductal neoplasia and prolongs survival in a murine model of pancreatic cancer. J Gastrointest Surg 2012; 16: 104–112 discussion 112.
Rabinowitz JD, White E . Autophagy and metabolism. Science 2010; 330: 1344–1348.
Hsu PP, Sabatini DM . Cancer cell metabolism: Warburg and beyond. Cell 2008; 134: 703–707.
Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci USA 2012; 109: 7031–7036.
Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 2004; 113: 1641–1650.
Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B . Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J 2002; 367: 729–740.
Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. Embo J 2007; 26: 1129–1139.
Praus M, Wauterickx K, Collen D, Gerard RD . Reduction of tumor cell migration and metastasis by adenoviral gene transfer of plasminogen activator inhibitors. Gene Ther 1999; 6: 227–236.
Acknowledgements
We dedicate this manuscript to the memory of Angelika Bierhaus, PhD, our friend and benefactor. She was a pioneering RAGE biologist, generous and kind in her dealings with colleagues, who provided us the mice with which we completed this work, and who sadly passed away on 15 April 2012 after a long and courageous battle with cancer. Angelika had a great love of life and she was generous, kind and warm hearted. She was always full of plans for scientific endeavors, even when her disease began to take its toll. She steadfastly denied that she would be defeated, recently celebrating her 50th birthday; however, she was aware that her remaining time was short. She dedicated herself to research, but despite her incredible strength, she was not able to overcome the disease, which tragically took her life.We thank Christine Heiner (University of Pittsburgh. Department of Surgery) for her critical reading of the manuscript. This project was supported by grants from the National Institutes of Health (P01 CA 101944 to MTL, R01CA160417 to DT) and the University of Pittsburgh (DT and HJZ). This project used University of Pittsburgh Cancer Institute shared resources that are supported in part by award P30CA047904. We also thank Dr Donna Stolz and Simon Watkins for providing the confocal microscopy facilities at the Center for Biologic Imaging at University of Pittsburgh School of Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kang, R., Tang, D., Schapiro, N. et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 33, 567–577 (2014). https://doi.org/10.1038/onc.2012.631
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.631
Keywords
This article is cited by
-
Potential antiviral activities of chrysin against hepatitis B virus
Gut Pathogens (2023)
-
Flavinated SDHA underlies the change in intrinsic optical properties of oral cancers
Communications Biology (2023)
-
Crosstalk between ferroptosis and macrophages: potential value for targeted treatment in diseases
Molecular and Cellular Biochemistry (2023)
-
Lipopolysaccharides induce a RAGE-mediated sensitization of sensory neurons and fluid hypersecretion in the upper airways
Scientific Reports (2021)
-
AGE-RAGE synergy influences programmed cell death signaling to promote cancer
Molecular and Cellular Biochemistry (2021)