Abstract
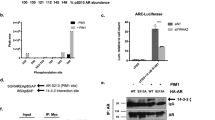
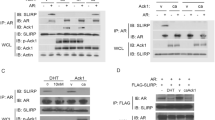
We have recently identified apoptosis-antagonizing transcription factor (AATF), tumor-susceptibility gene 101 (TSG101) and zipper-interacting protein kinase (ZIPK) as novel coactivators of the androgen receptor (AR). The mechanisms of coactivation remained obscure, however. Here we investigated the interplay and interdependence between these coactivators and the AR using the endogenous prostate specific antigen (PSA) gene as model for AR-target genes. Chromatin immunoprecipitation in combination with siRNA-mediated knockdown revealed that recruitment of AATF and ZIPK to the PSA enhancer was dependent on AR, whereas recruitment of TSG101 was dependent on AATF. Association of AR and its coactivators with the PSA enhancer or promoter occurred in cycles. Dissociation of AR-transcription complexes was due to degradation because inhibition of the proteasome system by MG132 caused accumulation of AR at enhancer/promoter elements. Moreover, inhibition of degradation strongly reduced transcription, indicating that continued and efficient transcription is based on initiation, degradation and reinitiation cycles. Interestingly, knockdown of ZIPK by siRNA had a similar effect as MG132, leading to reduced transcription but enhanced accumulation of AR at androgen-response elements. In addition, knockdown of ZIPK, as well as overexpression of a dominant-negative ZIPK mutant, diminished polyubiquitination of AR. Furthermore, ZIPK cooperated with the E3 ligase Mdm2 in AR-dependent transactivation, assembled into a single complex on chromatin and phosphorylated Mdm2 in vitro. These results suggest that ZIPK has a crucial role in regulation of ubiquitination and degradation of the AR, and hence promoter clearance and efficient transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heemers HV, Tindall DJ . Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007; 28: 778–808.
Faus H, Haendler B . Post-translational modifications of steroid receptors. Biomed Pharmacother 2006; 60: 520–528.
Gioeli D, Paschal BM . Post-translational modification of the androgen receptor. Mol Cell Endocrinol 2012; 352: 70–78.
Hinojos CA, Sharp ZD, Mancini MA . Molecular dynamics and nuclear receptor function. Trends Endocrinol Metab 2005; 16: 12–18.
Johnsen SA, Kangaspeska S, Reidt G, Gannon F . Interfering with the dynamics of estrogen receptor-regulated transcription. Ernst Schering Found Symp Proc 2006; 1: 1–12.
Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M . Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 2000; 103: 843–852.
Reid G, Hubner MR, Métivier R, Brand H, Denger S, Manu D et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 2003; 11: 695–707.
Métivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 2003; 115: 751–763.
Wang Q, Carroll JS, Brown M . Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 2005; 19: 631–642.
Kang Z, Pirskanen A, Janne OA, Palvimo JJ . Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 2002; 277: 48366–48371.
Kang Z, Janne OA, Palvimo JJ . Coregulator recruitment and histone modifications in transcriptional regulation by the androgen receptor. Mol Endocrinol 2004; 18: 2633–2648.
Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N et al. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 2009; 69: 958–966.
Keppler BR, Archer TK, Kinyamu HK . Emerging roles of the 26S proteasome in nuclear hormone receptor-regulated transcription. Biochim Biophys Acta 2011; 1809: 109–118.
Gaughan L, Logan IR, Neal DE, Robson CN . Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res 2005; 33: 13–26.
Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell 2009; 15: 270–282.
Leister P, Burgdorf S, Scheidtmann KH . Apoptosis antagonizing transcription factor AATF is a novel co-activator of nuclear hormone receptors. Signal Transduction 2003; 3: 17–25.
Burgdorf S, Leister P, Scheidtmann KH . TSG101 interacts with apoptosis-antagonizing transcription factor and enhances androgen receptor-mediated transcription by promoting its monoubiquitination. J Biol Chem 2004; 279: 17524–17534.
Leister P, Felten A, Chasan AI, Scheidtmann KH . ZIP kinase plays a crucial role in androgen receptor-mediated transcription. Oncogene 2008; 27: 3292–3300.
Page G, Lodige I, Kogel D, Scheidtmann KH . AATF, a novel transcription factor that interacts with Dlk/ZIP kinase and interferes with apoptosis. FEBS Lett 1999b; 462: 187–191.
Passananti C, Floridi A, Fanciulli M . Che-1/AATF, a multivalent adaptor connecting transcriptional regulation, checkpoint control, and apoptosis. Biochem Cell Biol 2007; 85: 477–483.
Bruno T, De Angelis R, De Nicola F, Barbato C, Di Padova M, Corbi N et al. Che-1 affects cell growth by interfering with the recruitment of HDAC1 by Rb. Cancer Cell 2002; 2: 387–399.
Di Padova M, Bruno T, De Nicola F, Iezzi S, D'Angelo C, Gallo R et al. Che-1 arrests human colon carcinoma cell proliferation by displacing HDAC1 from the p21WAF1/CIP1 promoter. J Biol Chem 2003; 278: 36496–36504.
Scheidtmann KH . Dlk/ZIP kinase, a novel Ser/Thr-specific protein kinase with multiple functions. Signal Transduction 2007; 7: 248–259.
Brognard J, Zhang YW, Puto LA, Hunter T . Cancer-associated loss-of-function mutations implicate DAPK3 as a tumor-suppressing kinase. Cancer Res 2011; 71: 3152–3161.
Bi J, Lau SH, Hu L, Rao HL, Liu HB, Zhan WH et al. Downregulation of ZIP kinase is associated with tumor invasion, metastasis and poor prognosis in gastric cancer. Int J Cancer 2009; 124: 1587–1593.
Natrajan R, Mackay A, Lambros MB, Weigelt B, Wilkerson PM, Manie E et al. A whole-genome massively parallel sequencing analysis of BRCA1 mutant oestrogen receptor-negative and -positive breast cancers. J Pathol 2012; 227: 29–41.
Kawai T, Matsumoto M, Takeda K, Sanjo H, Akira S . ZIP kinase, a novel serine/threonine kinase which mediates apoptosis. Mol Cell Biol 1998; 18: 1642–1651.
Sato N, Kawai T, Sugiyama K, Muromoto R, Imoto S, Sekine Y et al. Physical and functional interactions between STAT3 and ZIP kinase. Int Immunol 2005; 17: 1543–1552.
Engemann H, Heinzel V, Page G, Preuss U, Scheidtmann KH . DAP-like kinase interacts with the rat homolog of Schizosaccharomyces pombe CDC5 protein, a factor involved in pre-mRNA splicing and required for G2/M phase transition. Nucleic Acids Res 2002; 30: 1408–1417.
De Nicola F, Bruno T, Iezzi S, Di Padova M, Floridi A, Passananti C et al. The prolyl isomerase Pin1 affects Che-1 stability in response to apoptotic DNA damage. J Biol Chem 2007; 282: 19685–19691.
Li L, Liao J, Ruland J, Mak TW, Cohen SNA . TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control. Proc Natl Acad Sci USA 2001; 98: 1619–1624.
Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C . Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J 2002b; 21: 4037–4048.
Burch LR, Scott M, Pohler E, Meek D, Hupp T . Phage-peptide display identifies the interferon-responsive, death-activated protein kinase family as a novel modifier of MDM2 and p21WAF1. J Mol Biol 2004; 337: 115–128.
Saji S, Okumura N, Eguchi H, Nakashima S, Suzuki A, Toi M et al. MDM2 enhances the function of estrogen receptor alpha in human breast cancer cells. Biochem Biophys Res Commun 2001; 281: 259–265.
Sharma D, Fondell JD . Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci USA 2002; 99: 7934–7939.
Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C . Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D3. J Mol Biol 2005; 350: 65–77.
Lin HK, Altuwaijri S, Lin WJ, Kan PY, Collins LL, Chang C . Proteasome activity is required for androgen receptor transcriptional activity via regulation of androgen receptor nuclear translocation and interaction with coregulators in prostate cancer cells. J Biol Chem 2002a; 277: 36570–36576.
Chymkowitch P, Le May N, Charneau P, Compe E, Egly JM . The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J 2011; 30: 468–479.
Graves PR, Winkfield KM, Haystead TA . Regulation of zipper-interacting protein kinase activity in vitro and in vivo by multisite phosphorylation. J Biol Chem 2005; 280: 9363–9374.
Felten A, Leister P, Burgdorf S, Uhlmann L, Scheidtmann KH . Characterization of rat BLOS2/Ceap, a putative yeast She3 homolog, as interaction partner of apoptosis antagonizing transcription factor/Che-1. Biol Chem 2007; 388: 569–582.
Acknowledgements
We thank Dr Klemens Rottner and Dr Walter Witke from the Institute of Genetics (Bonn, Germany) for their hospitality during revision and Dr Maurizio Fanciulli (Regina Elena Cancer Institute, Rome, Italy) for antibodies against AATF/Che-1. This work was supported by the German Research Association to KHS (Sche246/20–1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Felten, A., Brinckmann, D., Landsberg, G. et al. Zipper-interacting protein kinase is involved in regulation of ubiquitination of the androgen receptor, thereby contributing to dynamic transcription complex assembly. Oncogene 32, 4981–4988 (2013). https://doi.org/10.1038/onc.2012.503
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.503