Abstract
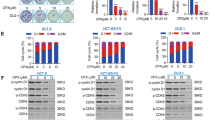
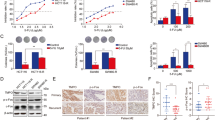
5-Fluorouracil (5-FU) is an anti-metabolite that is in clinical use for treatment of several cancers. In cells, it is converted into three distinct fluoro-based nucleotide analogs, which interfere with DNA synthesis and repair, leading to genome impairment and, eventually, apoptotic cell death. Current knowledge states that in certain cell types, 5-FU-induced stress is signaling through a p53-dependent induction of tumor necrosis factor-receptor oligomerization required for death-inducing signaling complex formation and caspase-8 activation. Here we establish a role of calcium (Ca2+) as a messenger for p53 activation in response to 5-FU. Using a combination of pharmacological and genetic approaches, we show that treatment of colon carcinoma cells stimulates entry of extracellular Ca2+ through long lasting-type plasma membrane channels, which further directs posttranslational phosphorylation of at least three p53 serine residues (S15, S33 and S37) by means of calmodulin (CaM) activity. Obstructing this pathway by the Ca2+-chelator BAPTA (1,2-bis(o-aminophenoxy)ethane- N,N,N',N'-tetraacetic acid) or by inhibitors of CaM efficiently reduces 5-FU-induced caspase activities and subsequent cell death. Moreover, ectopic expression of p53 S15A in HCT116 p53−/− cells confirmed the importance of a Ca2+–CaM–p53 axis in 5-FU-induced extrinsic apoptosis. The fact that a widely used therapeutic drug, such as 5-FU, is operating via this pathway could provide new therapeutic intervention points, or specify new combinatorial treatment regimes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- Ca2+:
-
calcium
- DISC:
-
death-inducing signaling complex
- 5-FU:
-
5-fluorouracil.
References
Longley DB, Harkin DP, Johnston PG . 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–338.
Kufe DW, Major PP . 5-Fluorouracil incorporation into human breast carcinoma RNA correlates with cytotoxicity. J Biol Chem 1981; 256: 9802–9805.
Glazer RI, Lloyd LS . Association of cell lethality with incorporation of 5-fluorouracil and 5-fluorouridine into nuclear RNA in human colon carcinoma cells in culture. Mol Pharmacol 1982; 21: 468–473.
Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest 1999; 104: 263–269.
Derheimer FA, O’Hagan HM, Krueger HM, Hanasoge S, Paulsen MT, Ljungman M . RPA and ATR link transcriptional stress to p53. Proc Natl Acad Sci USA 2007; 104: 12778–12783.
Matuo R, Sousa FG, Escargueil AE, Grivicich I, Garcia-Santos D, Chies JA et al. 5-Fluorouracil and its active metabolite FdUMP cause DNA damage in human SW620 colon adenocarcinoma cell line. J Appl Toxicol 2009; 29: 308–316.
Seiple L, Jaruga P, Dizdaroglu M, Stivers JT . Linking uracil base excision repair and 5-fluorouracil toxicity in yeast. Nucleic Acids Res 2006; 34: 140–151.
Olsson M, Zhivotovsky B . Caspases and cancer. Cell Death Differ 2011; 18: 1441–1449.
O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res 1997; 57: 4285–4300.
Perraud A, Akil H, Nouaille M, Petit D, Labrousse F, Jauberteau MO et al. Expression of p53 and DR5 in normal and malignant tissues of colorectal cancer: correlation with advanced stages. Oncol Rep 2011; 26: 1091–1097.
Wang S, El-Deiry WS . Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res 2004; 64: 6666–6672.
Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 2006; 25: 838–848.
Borralho PM, Moreira da Silva IB, Aranha MM, Albuquerque C, Nobre LC, Steer CJ et al. Inhibition of Fas expression by RNAi modulates 5-fluorouracil-induced apoptosis in HCT116 cells expressing wild-type p53. Biochim Biophys Acta 2007; 1772: 40–47.
Henry RE, Andrysik Z, Paris R, Galbraith MD, Espinosa JM . A DR4:tBID axis drives the p53 apoptotic response by promoting oligomerization of poised BAX. EMBO J 2012; 31: 1266–1278.
Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA . Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res 2002; 62: 5800–5806.
Olsson M, Vakifahmetoglu H, Abruzzo PM, Hogstrand K, Grandien A, Zhivotovsky B . DISC-mediated activation of caspase-2 in DNA damage-induced apoptosis. Oncogene 2009; 28: 1949–1959.
Longley DB, Allen WL, McDermott U, Wilson TR, Latif T, Boyer J et al. The roles of thymidylate synthase and p53 in regulating Fas-mediated apoptosis in response to antimetabolites. Clin Cancer Res 2004; 10: 3562–3571.
Oberle C, Huai J, Reinheckel T, Tacke M, Rassner M, Ekert PG et al. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ 2010; 17: 1167–1178.
Kaeser MD, Pebernard S, Iggo RD . Regulation of p53 stability and function in HCT116 colon cancer cells. J Biol Chem 2004; 279: 7598–7605.
Harr MW, Distelhorst CW . Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb Perspect Biol 2010; 2: a005579.
Orrenius S, Zhivotovsky B, Nicotera P . Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–565.
Berridge MJ . The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 2002; 32: 235–249.
Maclaine NJ, Hupp TR . How phosphorylation controls p53. Cell Cycle 2011; 10: 916–921.
Coutinho I, Pereira G, Leao M, Goncalves J, Corte-Real M, Saraiva L . Differential regulation of p53 function by protein kinase C isoforms revealed by a yeast cell system. FEBS Lett 2009; 583: 3582–3588.
Lavin MF, Gueven N . The complexity of p53 stabilization and activation. Cell Death Differ 2006; 13: 941–950.
Pospisilova S, Brazda V, Kucharikova K, Luciani MG, Hupp TR, Skladal P et al. Activation of the DNA-binding ability of latent p53 protein by protein kinase C is abolished by protein kinase CK2. Biochem J 2004; 378 (Pt 3): 939–947.
Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A . DAP kinase activates a p19ARF/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nat Cell Biol 2001; 3: 1–7.
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005; 18: 283–293.
Craig AL, Chrystal JA, Fraser JA, Sphyris N, Lin Y, Harrison BJ et al. The MDM2 ubiquitination signal in the DNA-binding domain of p53 forms a docking site for calcium calmodulin kinase superfamily members. Mol Cell Biol 2007; 27: 3542–3555.
Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 2000; 287: 1824–1827.
Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 1998; 281: 1674–1677.
de la Cruz-Morcillo MA, Valero ML, Callejas-Valera JL, Arias-Gonzalez L, Melgar-Rojas P, Galan-Moya EM et al. P38MAPK is a major determinant of the balance between apoptosis and autophagy triggered by 5-fluorouracil: implication in resistance. Oncogene 2012; 31: 1073–1085.
Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J et al. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep 2004; 5: 161–166.
Kim HD, Kim TS, Kim J . Aberrant ribosome biogenesis activates c-Myc and ASK1 pathways resulting in p53-dependent G1 arrest. Oncogene 2011; 30: 3317–3327.
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R . Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008; 27: 6407–6418.
Lamprecht SA, Lipkin M . Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003; 3: 601–614.
Kumar SK, Goldberg RM . Adjuvant chemotherapy for colon cancer. Curr Oncol Rep 2001; 3: 94–101.
Burger K, Muhl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M et al. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem 2010; 285: 12416–12425.
Li Y, Yang DQ . The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther 2010; 9: 113–125.
Bagnoli M, Canevari S, Mezzanzanica D . Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol 2010; 42: 210–213.
Stagni V, Mingardi M, Santini S, Giaccari D, Barila D . ATM kinase activity modulates cFLIP protein levels: potential interplay between DNA damage signalling and TRAIL-induced apoptosis. Carcinogenesis 2010; 31: 1956–1963.
Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG . Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther 2005; 4: 2026–2036.
Abdulghani J, El-Deiry WS . TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets 2010; 14: 1091–1108.
Acknowledgements
We thank Professors Bert Vogelstein, Peter H Krammer, Ladislav Anděra, Moshe Oren and Dr Inna Lavrik for their contribution of cells, antibodies and vectors. This study was supported by grants from the Swedish Science Foundation, the Swedish and Stockholm Cancer Societies, the Swedish Childhood Cancer Foundation and the EC-FP-6 (Chemores) and EC-FP-7 (APO-SYS). MO is a fellow of the Swedish Society of Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Can, G., Akpinar, B., Baran, Y. et al. 5-Fluorouracil signaling through a calcium–calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene 32, 4529–4538 (2013). https://doi.org/10.1038/onc.2012.467
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.467
Keywords
This article is cited by
-
Expression of molecular markers and synergistic anticancer effects of chemotherapy with antimicrobial peptides on glioblastoma cells
Cancer Chemotherapy and Pharmacology (2024)
-
Targeting oncogenic Notch signaling with SERCA inhibitors
Journal of Hematology & Oncology (2021)
-
5-Flurouracil disrupts nuclear export and nuclear pore permeability in a calcium dependent manner
Apoptosis (2017)
-
Multi-omics landscapes of colorectal cancer subtypes discriminated by an individualized prognostic signature for 5-fluorouracil-based chemotherapy
Oncogenesis (2016)
-
Gene expression profile analysis of Manila clam (Ruditapes philippinarum) hemocytes after a Vibrio alginolyticus challenge using an immune-enriched oligo-microarray
BMC Genomics (2014)