Abstract
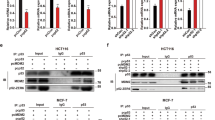
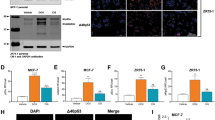
The p53 tumor suppressor protein is frequently mutated in human tumors. It is thought that the p53 pathway is indirectly impaired in the remaining tumors, for example by overexpression of its important regulators Mdm2 and Mdm4, making them attractive targets for the development of anti-cancer agents. Recent studies have suggested that Mdm4 levels determine the sensitivity of tumor cells for anti-cancer therapy. To investigate this possibility, we studied the drug sensitivity of several breast cancer cell lines containing wild-type p53, but expressing different Mdm4 levels. We show that endogenous Mdm4 levels can affect the sensitivity of breast cancer cells to anti-cancer agents, but in a cell line-dependent manner and depending on an intact apoptotic response. Furthermore, treatment with the non-genotoxic agent Nutlin-3 sensitizes cells for doxorubicin, showing that activation of p53 by targeting its regulators is an efficient strategy to decrease cell viability of breast cancer cells. These results confirm a function of Mdm4 in determining the efficacy of chemotherapeutic agents to induce apoptosis of cancer cells in a p53-dependent manner, although additional undetermined factors also influence the drug response. Targeting Mdm4 to sensitize tumor cells for chemotherapeutic drugs might be a strategy to effectively treat tumors harboring wild-type p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S et al. (2006). MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther 5: 2358–2365.
Brooks CL, Gu W . (2006). p53 Ubiquitination: Mdm2 and beyond. Mol Cell 21: 307–315.
Brummelkamp TR, Bernards R, Agami R . (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553.
Cao C, Shinohara ET, Subhawong TK, Geng L, Woon KK, Albert JM et al. (2006). Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol Cancer Ther 5: 411–417.
Carlotti F, Bazuine M, Kekarainen T, Seppen J, Pognonec P, Maassen JA et al. (2004). Lentiviral vectors efficiently transduce quiescent mature 3T3-L1 adipocytes. Mol Ther 9: 209–217.
Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E et al. (2006). MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood 107: 4109–4114.
Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R et al. (2004). Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol Cell Biol 24: 5835–5843.
de Graaf P, Little NA, Ramos YF, Meulmeester E, Letteboer SJ, Jochemsen AG . (2003). Hdmx protein stability is regulated by the ubiquitin ligase activity of Mdm2. J Biol Chem 278: 38315–38324.
Fuster JJ, Sanz-Gonzalez SM, Moll UM, Andres V . (2007). Classic and novel roles of p53: prospects for anticancer therapy. Trends Mol Med 13: 192–199.
Haupt Y, Maya R, Kazaz A, Oren M . (1997). Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299.
Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M et al. (2009). Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat (in press).
Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J . (2006). MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem 281: 33030–33035.
Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M et al. (2004). Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10: 1321–1328.
Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F . (2008). E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene 27: 5303–5314.
Kubbutat MH, Jones SN, Vousden KH . (1997). Regulation of p53 stability by Mdm2. Nature 387: 299–303.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. (2006). Inactivation of the p53 pathway in retinoblastoma. Nature 444: 61–66.
Meulmeester E, Pereg Y, Shiloh Y, Jochemsen AG . (2005). ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation. Cell Cycle 4: 1166–1170.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ . (1992). The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69: 1237–1245.
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M et al. (1995). Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43.
Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B . (1992). Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358: 80–83.
Oliver FJ, de la RG, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM . (1998). Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem 273: 33533–33539.
Oruetxebarria I, Venturini F, Kekarainen T, Houweling A, Zuijderduijn LM, Mohd-Sarip A et al. (2004). P16INK4a is required for hSNF5 chromatin remodeler-induced cellular senescence in malignant rhabdoid tumor cells. J Biol Chem 279: 3807–3816.
Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW . (2006). Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer Res 66: 3169–3176.
Pereg Y, Lam S, Teunisse A, Biton S, Meulmeester E, Mittelman L et al. (2006). Differential roles of ATM- and Chk2-mediated phosphorylations of Hdmx in response to DNA damage. Mol Cell Biol 26: 6819–6831.
Pereg Y, Shkedy D, de Graaf P, Meulmeester E, Edelson-Averbukh M, Salek M et al. (2005). Phosphorylation of Hdmx mediates its Hdm2- and ATM-dependent degradation in response to DNA damage. Proc Natl Acad Sci USA 102: 5056–5061.
Ramos YF, Stad R, Attema J, Peltenburg LT, van der Eb AJ, Jochemsen AG . (2001). Aberrant expression of HDMX proteins in tumor cells correlates with wild-type p53. Cancer Res 61: 1839–1842.
Riemenschneider MJ, Buschges R, Wolter M, Reifenberger J, Bostrom J, Kraus JA et al. (1999). Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res 59: 6091–6096.
Riemenschneider MJ, Knobbe CB, Reifenberger G . (2003). Refined mapping of 1q32 amplicons in malignant gliomas confirms MDM4 as the main amplification target. Int J Cancer 104: 752–757.
Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S et al. (2008). Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA 105: 3933–3938.
Shapiro CL, Recht A . (2001). Side effects of adjuvant treatment of breast cancer. N Engl J Med 344: 1997–2008.
Shvarts A, Bazuine M, Dekker P, Ramos YF, Steegenga WT, Merckx G et al. (1997). Isolation and identification of the human homolog of a new p53-binding protein, Mdmx. Genomics 43: 34–42.
Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M et al. (1996). MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J 15: 5349–5357.
Stad R, Little NA, Xirodimas DP, Frenk R, van der Eb AJ, Lane DP et al. (2001). Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep 2: 1029–1034.
Stad R, Ramos YF, Little N, Grivell S, Attema J, Der Eb AJ et al. (2000). Hdmx stabilizes Mdm2 and p53. J Biol Chem 275: 28039–28044.
Tewari M, Quan LT, O'rourke K, Desnoyers S, Zeng Z, Beidler DR et al. (1995). Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81: 801–809.
Toledo F, Wahl GM . (2006). Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6: 909–923.
Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H et al. (2006). Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA 103: 1888–1893.
van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D et al. (2003). Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. (2004). in vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848.
Wade M, Rodewald LW, Espinosa JM, Wahl GM . (2008). BH3 activation blocks Hdmx suppression of apoptosis and cooperates with Nutlin to induce cell death. Cell Cycle 7: 1973–1982.
Wade M, Wong ET, Tang M, Stommel JM, Wahl GM . (2006). Hdmx modulates the outcome of p53 activation in human tumor cells. J Biol Chem 281: 33036–33044.
Wasielewski M, Elstrodt F, Klijn JG, Berns EM, Schutte M . (2006). Thirteen new p53 gene mutants identified among 41 human breast cancer cell lines. Breast Cancer Res Treat 99: 97–101.
Acknowledgements
We thank Dr G Selivanova for providing RITA, Dr A Levine and Dr G Peters for providing anti-Mdm2 and anti-p16 antibodies, respectively. This study was supported by grants from the Association for International Cancer Research (grant 05-273) and by EC FP6 funding (contract 503576) to AG Jochemsen. This publication reflects the authors’ views and not necessarily those of the European Community. The EC is not liable for any use that may be made of the information contained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Lam, S., Lodder, K., Teunisse, A. et al. Role of Mdm4 in drug sensitivity of breast cancer cells. Oncogene 29, 2415–2426 (2010). https://doi.org/10.1038/onc.2009.522
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.522
Keywords
This article is cited by
-
Targeting p53–MDM2 interaction by small-molecule inhibitors: learning from MDM2 inhibitors in clinical trials
Journal of Hematology & Oncology (2022)
-
Model-based optimization of combination protocols for irradiation-insensitive cancers
Scientific Reports (2020)
-
DNA damage triggers tubular endoplasmic reticulum extension to promote apoptosis by facilitating ER-mitochondria signaling
Cell Research (2018)
-
Heterozygous p53V172F mutation in cisplatin-resistant human tumor cells promotes MDM4 recruitment and decreases stability and transactivity of p53
Oncogene (2016)
-
The Zn-finger domain of MdmX suppresses cancer progression by promoting genome stability in p53-mutant cells
Oncogenesis (2016)