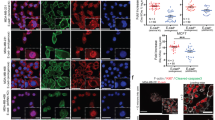
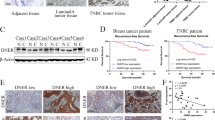
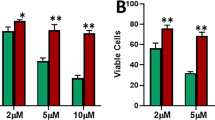
Abstract
The ERα signaling pathway is one of the most important and most studied pathways in human breast cancer, yet numerous questions still exist such as how hormonally responsive cancers progress to a more aggressive and hormonally independent phenotype. We have noted that human breast cancers exhibit a strong direct correlation between ERα and E-cadherin expression by immunohistochemistry, suggesting that ERα signaling might regulate E-cadherin and implying that this regulation might influence epithelial–mesenchymal transition (EMT) and tumor progression. To investigate this hypothesis and the mechanisms behind it, we studied the effects of ERα signaling in ERα-transfected ERα-negative breast carcinoma cell lines, the MDA-MB-468 and the MDA-MB-231 and the effects of ERα knockdown in naturally expressing ERα-positive lines, MCF-7 and T47D. When ERα was overexpressed in the ERα-negative lines, 17β-estradiol (E2) decreased slug and increased E-cadherin. Clones maximally exhibiting these changes grew more in clumps and became less invasive in Matrigel. When ERα was knocked down in the ERα-positive lines, slug increased, E-cadherin decreased, cells became spindly and exhibited increased Matrigel invasion. ERα signaling decreased slug expression by two different mechanisms: directly, by repression of slug transcription by the formation of a corepressor complex of ligand-activated ERα, HDAC inhibitor (HDAC1), and nuclear receptor corepressor (N-CoR) that bound the slug promoter in three half-site estrogen response elements (EREs); indirectly by phosphorylation and inactivation of GSK-3β through phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt). The GSK-3β inactivation, in turn, repressed slug expression and increased E-cadherin. In human breast cancer cases, there was a strong inverse correlation between slug and ERα and E-cadherin immunoreactivity. Our findings indicate that ERα signaling through slug regulates E-cadherin and EMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Alpaugh ML, Tomlinson JS, Kasraeian S, Barsky SH . (2002a). Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene 21: 3631–3643.
Alpaugh ML, Tomlinson JS, Ye Y, Barsky SH . (2002b). Relationship of sialyl-lewisx/a underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Path 161: 619–628.
Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM . (2005). Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial–mesenchymal transition. J Cell Biol 168: 29–33.
Barsky SH . (2003). Myoepithelial mRNA expression profiling reveals a common tumor suppressor phenotype. Exp Mol Pathol 74: 113–122.
Berx G, Van Roy F . (2001). The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Can Res 3: 289–293.
Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F . (2004). Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci 25: 363–373.
Catalano A, Rodilossi S, Rippo MR, Caprari P, Procopio A . (2004). Induction of stem cell factor/c-Kit/Slug signal transduction in multidrug-resistant malignant mesothelioma cells. J Biol Chem 279: 46706–46714.
Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C et al. (2006). Snai1 and slug play distinct roles during breast carcinoma progression. Clin Cancer Res 12: 5395–5402.
de Groot RP, Auwerx J, Bourouis M, Sassone-Corsi P . (1993). Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene 8: 841–847.
Dhasarathy A, Kajita M, Wade PA . (2007). The transcription factor snai1 mediates epithelial to mesenchymal transitions by repression of estrogen receptor-α. Mol Endocrinol 21: 2907–2918.
Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisani OM . (1994). A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J Biol Chem 269: 32187–32193.
Frame S, Cohen P . (2001). GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359: 1–16.
Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA . (2003). MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113: 207–219.
Grisouard J, Medunjanin S, Hermani A, Shukla A, Mayer D . (2007). Glycogen synthase kinase-3 protects estrogen receptor α from proteosomal degradation and is required for full transcriptional activity of the receptor. Mol Endocrinol 21: 2427–2439.
Hajra KM, Chen DY, Fearon ER . (2002). The SLUG zinc-finger protein represses E-Cadherin in breast cancer. Cancer Res 62: 1613–1618.
Hall JM, McDonnell DP . (2005). Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv 5: 343–357.
Hayashida Y, Honda K, Idogawa M, Ino Y, Ono M, Tsuchida A et al. (2005). E-cadherin regulates the association between β-catenin and actinin-4. Cancer Res 65: 8836–8845.
Hiraguri S, Godfrey T, Nakamura H, Graff J, Collins C, Shayesteh L et al. (1998). Mechanisms of inactivation of E-cadherin in breast cancer cell lines. Cancer Res 58: 1972–1977.
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR . (2000). Requirement for glycogen synthase kinase-3 in cell survival and NF-κB activation. Nature 406: 86–90.
Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R et al. (1995). Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377: 397–404.
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG . (2007). Epithelial to mesenchymal transition: expression of the regulators snai1, slug and twist in pancreatic cancer. Clin Cancer Res 13: 4769–4776.
Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettg ER, Tong HL et al. (1996). Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res 56: 3954–3960.
Joshi AS, Sharangpani GM, Porter K, Keyhani S, Morrison C, Basu AS et al. (2007). Semi-automated imaging system to quantitate Her-2/neu membrane receptor immunoreactivity in human breast cancer. Cytometry Part A 71A: 273–285.
Kato S, Masuhiro Y, Watanabe M, Kobayashi Y, Takeyama K, Endoh H et al. (2000). Molecular mechanism of a cross-talk between oestrogen and growth factor signaling pathways. Genes Cells 5: 593–601.
Keeton EK, Brown M . (2005). Cell cycle progression stimulated by tamoxifen-bound estrogen receptor- and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol 19: 1543–1554.
Lacroix M, Leclercq G . (2004). Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Can Res Treat 83: 249–289.
Lombaerts M, Van Wezel T, Philippo K, Dierssen JWF, Zimmerman RME, Oosting J et al. (2006). E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 94: 661–671.
Medunjanin S, Hermani A, De Servil B, Grisouard J, Rincke G, Mayer D . (2005). Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor and is involved in the regulation of receptor activity. J Biol Chem 280: 33006–33014.
Mendez P, Garcia-Segura LM . (2006). Phosphatidylinositol 3-Kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology 147: 3027–3039.
Moggs JG, Murphy TC, Lim FL, Moore DJ, Stuckey R, Antrobus K et al. (2005). Anti-proliferative effect of estrogen in breast cancer cells that re-express ERα is mediated by aberrant regulation of cell cycle genes. J Mol Endocrinol 34: 535–551.
Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S et al. (2006). Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for snai1, slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res 66: 9543–9556.
Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN . (2000). Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc Natl Acad Sci USA 97: 10972–10977.
Nam JS, Kang MJ, Suchar AM, Shimamura T, Kohn EA, Michalowska AM et al. (2006). Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res 66: 7176–7184.
Natsugoe S, Uchikado Y, Okumura H, Setoyama T, Matsumoto M, Setoyama T et al. (2007). Snai1 plays a key role in E-cadherin preserved esophageal squamous cell carcinoma. Oncol Rep 17: 517–523.
Nilsson S, Makea S, Treuter E, Tujague M, Thomsen J, Andersson G et al. (2001). Mechanisms of estrogen action. Physiol Rev 81: 1535–1565.
Oesterreich S, Deng W, Jiang S, Cui X, Ivanova M, Schiff R et al. (2003). Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res 63: 5203–5208.
Park KJ, Krishnan V, O'Malley BM, Yamamoto Y, Gaynor RB . (2005). Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell 18: 71–82.
Perrais M, Chen X, Perez-Moreno M, Gumbiner BM . (2007). E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 18: 2013–2025.
Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK . (2001). Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor snai1. J Biol Chem 276: 24661–24666.
Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR . (2004). E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 23: 1739–1748.
Roll JD, Rivenback AG, Jones WD, Coleman WB . (2008). DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol Cancer 7: 15.
Sasaki C, Lin H, Morin PJ, Longo DL . (2000). Truncation of the extracellular region abrogates cell contact but retains the growth-suppressive activity of E-cadherin. Cancer Res 60: 7057–7065.
Sharangpani GM, Joshi AS, Porter K, Deshpande AS, Keyhani S, Nail GA et al. (2007). Semi-automated imaging system to quantitate estrogen and progesterone receptor immunoreactivity in human breast cancer. J Microscopy 226: 244–255.
Tomlinson JS, Alpaugh ML, Barsky SH . (2001). An intact overexpressed E-cadherin/α,β-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res 61: 5231–5241.
Tora L, White J, Brou C, Tasset D, Webster N, Scheer E et al. (1989). The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59: 477–487.
Turenne GA, Price BD . (2001). Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53's transcriptional activity. BMC Cell Biol 2: 12–20.
Uchikado Y, Natsugoe S, Okumura H, Setoyama T, Matsumoto M, Ishigami S et al. (2005). Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 11: 1174–1180.
Voss TC, Demarco IA, Booker CF, Day RN . (2005). Corepressor subnuclear organization is regulated by estrogen receptor via a mechanism that requires the DNA-binding domain. Mol Cell Endocrinol 231: 33–47.
Watcharasit P, Bijur GN, Son L, Zhu J, Chen X, Jope RS . (2003). Glycogen synthase kinase-3 (GSK3β) binds to and promotes the actions of p53. J Biol Chem 278: 48872–48879.
Wong AST, Gumbiner BM . (2003). Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 161: 1191–1203.
Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z et al. (2006). MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol 26: 7269–7282.
Yang S, Du J, Wang Z, Yuan W, Qiao Y, Zhang M et al. (2007). BMP-6 promotes E-cadherin expression through repressing δEF1 in breast cancer cells. BMC Cancer 7: 211.
Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX, Barsky SH . (2008). ERα suppresses slug expression directly by transcriptional repression. Biochem J 416: 179–187.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M et al. (2004). Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol 6: 931–940.
Acknowledgements
This study was supported by the Department of Defense Breast Cancer Research Program Grant W81XWH-06-1-0631, the Ohio State Strategic Initiative Grant Program and The Donald A Senhauser Endowment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Ye, Y., Xiao, Y., Wang, W. et al. ERα signaling through slug regulates E-cadherin and EMT. Oncogene 29, 1451–1462 (2010). https://doi.org/10.1038/onc.2009.433
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.433
Keywords
This article is cited by
-
PLCD3 inhibits apoptosis and promotes proliferation, invasion and migration in gastric cancer
Discover Oncology (2024)
-
The emerging role of ubiquitin-specific protease 20 in tumorigenesis and cancer therapeutics
Cell Death & Disease (2022)
-
Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis
Cellular and Molecular Bioengineering (2022)
-
CBP-mediated Slug acetylation stabilizes Slug and promotes EMT and migration of breast cancer cells
Science China Life Sciences (2021)
-
Novel 3D embryo implantation model within macroporous alginate scaffolds
Journal of Biological Engineering (2020)