Key Points
-
Testicular biopsy can be performed in children for diagnostic purposes or potentially for therapeutic reasons
-
Patients with gonadal dysgenesis and severe undermasculinization are at considerable risk of germ cell tumours
-
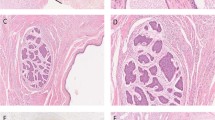
Carcinoma in situ or gonadoblastoma can be detected in the dysgenetic gonads of patients with disorders of sexual development using immunohistochemical markers for OCT-3/4, Kit ligand (SCF) and TSPY
-
Testicular biopsy performed on a prepubertal testicle does not lead to formation of antisperm antibodies or testicular microlithiasis
-
Testicular histology is of considerable value in the prediction of fertility potential in individuals with undescended testes
-
Cryopreservation of testicular tissue samples shows promise for the preservation of fertility in prepubertal boys who receive gonadotoxic chemotherapy
Abstract
No consensus exists regarding the precise role of testicular biopsy in prepubertal boys, although it is considered useful for assessing the potential consequences of undescended testes on fertility. Current scientific knowledge indicates that surgeons should broaden indications for this procedure. For example, the use of immunohistochemical markers such as OCT/3-4, TSPY, Kit ligand (SCF) and ALPP (PLAP) has considerably facilitated the detection of germ cell tumour precursors, such as carcinoma in situ and/or gonadoblastoma. These markers are very important for evaluating malignancy risk in undervirilized patients with 46,XY disorders of sexual development. Testicular histology is also of considerable value in the prediction of both fertility potential and risk of cancer in individuals with undescended testes, particularly those with intraabdominal undescended testes. New possibilities for the preservation of fertility after gonadotoxic chemotherapy — even for prepubertal boys — are emerging. Cryopreservation of testicular tissue samples for the preservation of fertility — although still an experimental method at present — is appealing in this context. In our opinion, testicular biopsy in prepubertal boys is a minor procedure that can provide valuable information for predicting the risk of malignancy and fertility, and might be useful in fertility preservation in the near future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hadziselimovic, F., Hecker, E. & Herzog, B. The value of testicular biopsy in cryptorchidism. Urol. Res. 12, 171–174 (1984).
Hadziselimovic, F. & Herzog, B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet 6, 1156–1157 (2001).
Skakkebaek, N. E. Possible carcinoma-in-situ of the testis. Lancet 9, 516–517 (1972).
Skakkebaek, N. E., Berthelsen, J. G., Giwercman, A. & Müller, J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 10, 19–28 (1987).
Van der Zwan, Y. G., Biermann, K., Wolffenbuttel, K. P., Cools, M. & Looijenga, L. H. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur. Urol. 67, 692–701 (2015).
McCann-Crosby, B. State of the art review in gonadal dysgenesis: challenges in diagnosis and management. Int. J. Pediatr. Endocrinol. 2014, 4 (2014).
Howell, S. & Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. North Am. 27, 927–943 (1998).
Wallace, W. H., Anderson, R. A. & Irvine, D. S. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 6, 209–218 (2005).
Keros, V. et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum. Reprod. 22, 1384–1395 (2007).
Wyns, C., Curaba, M., Vanabelle, B., Van Langendonckt, A. & Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update 16, 312–328 (2010).
Kvist, K. et al. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum. Reprod. 21, 484–491 (2006).
Gordon, D. L., Barr, A. B., Herrigel, J. E. & Paulsen, C. A. Testicular biopsy in man. I. Effect upon sperm concentration. Fertil. Steril. 16, 522–530 (1965).
Rowley, M. J., O'Keefe, K. B. & Heller, C. G. Decreases in sperm concentration due to testicular biopsy procedure in man. J. Urol. 101, 347–349 (1969).
Schoor, R. A., Elhanbly, S., Niederberger, C. S. & Ross, L. S. The role of testicular biopsy in the modern management of male infertility. J. Urol. 167, 197–200 (2002).
Dieckmann, K. P., Heinemann, V., Frey, U. & Pichlmeier, U. How harmful is contralateral testicular biopsy? — An analysis of serial imaging studies and a prospective evaluation of surgical complications. Eur. Urol. 48, 662–672 (2005).
Nistal, M., Paniagua, R., González-Peramato, P. & Reyes-Múgica, M. Perspectives in pediatric pathology, chapter 3. Testicular development from birth to puberty: systematic evaluation of the prepubertal testis. Pediatr. Dev. Pathol. 18, 173–186 (2015).
Giwercman, A., Berthelsen, J. G., Müller, J., von der Maase, H. & Skakkebaek, N. E. Screening for carcinoma-in-situ of the testis. Int. J. Androl. 10, 173–180 (1987).
Giwercman, A., Grindsted, J., Hansen, B., Jensen, O. M. & Skakkebaek, N. E. Testicular cancer risk in boys with maldescended testis: a cohort study. J. Urol. 138, 1214–1216 (1987).
van Casteren, N. J. et al. Heterogeneous distribution of ITGCNU in an adult testis: consequences for biopsy-based diagnosis. Int. J. Surg. Pathol. 16, 21–24 (2008).
Dieckmann, K. P., Kulejewski, M., Pichlmeier, U. & Loy, V. Diagnosis of contralateral testicular epithelial neoplasia (tin) in patients with testicular germ cell cancer: systematic two-site biopsies are more sensitive than a single random biopsy. Eur. Urol. 51, 175–183 (2006).
Møller, H., Cortes, D., Engholm, G. & Thorup, J. Risk of testicular cancer with cryptorchidism and with testicular biopsy: cohort study. BMJ 12, 729 (1998).
Patel, R. P. et al. Testicular microlithiasis and antisperm antibodies following testicular biopsy in boys with cryptorchidism. J. Urol. 174, 2008–2010 (2005).
Oosterhuis, J. W. & Looijenga, L. H. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 (2005).
Mandelbaum, S. L., Diamond, M. P. & DeCherney, A. H. The impact of antisperm antibodies on human infertility. J. Urol. 138, 1–8 (1987).
Mininberg, D. T., Chen, M. E. & Witkin, S. S. Antisperm antibodies in cryptorchid boys. Eur. J. Pediatr. 152, 23–24 (1993).
Cortes, D., Brandt, B. & Thorup, J. Direct mixed antiglobulin reaction (MAR) test in semen at follow-up after testicular biopsy of maldescended testes operated in puberty. Z. Kinderchir. 45, 227–228 (1990).
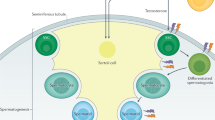
Cheng, C. Y. & Mruk, D. D. Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 (2002).
Furuya, S., Kumamoto, Y. & Sugiyama, S. Fine structure and development of Sertoli junctions in human testis. Arch. Androl. 1, 211–219 (1978).
Cheng, C. Y. & Mruk, D. D. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit. Rev. Biochem. Mol. Biol. 44, 245–263 (2009).
Looijenga, L. H. et al. Gonadal tumours and DSD. Best Pract. Res. Clin. Endocrinol. Metab. 24, 291–310 (2010).
Huyghe, E., Matsuda, T. & Thonneau, P. Increasing incidence of testicular cancer worldwide: a review. J. Urol. 170, 5–11 (2003).
Krausz, C. & Looijenga, L. H. Genetic aspects of testicular germ cell tumors. Cell Cycle 15, 3519–3524 (2008).
Li, Y. et al. The Y-encoded TSPY protein: a significant marker potentially plays a role in the pathogenesis of testicular germ cell tumors. Hum. Pathol. 38, 1470–1481 (2007).
Oram, S. W., Liu, X. X., Lee, T. L., Chan, W. Y. & Lau, Y. F. TSPY potentiates cell proliferation and tumorigenesis by promoting cell cycle progression in HeLa and NIH3T3 cells. BMC Cancer 9, 154 (2006).
Hersmus, R. et al. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J. Pathol. 215, 31–38 (2008).
Scully, R. E. Gonadoblastoma. A review of 74 cases. Cancer 25, 1340–1356 (1970).
Hoei-Hansen, C. E., Rajpert- De Meyts, E., Daugaard, G. & Skakkebaek, N. E. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann. Oncol. 16, 863–868 (2005).
Rørth, M. et al. Carcinoma in situ in the testis. Scand. J. Urol. Nephrol. 34 (Suppl.), 166–186 (2000).
Dieckmann, K. P. & Skakkebaek, N. E. Carcinoma in situ of the testis: review of biological and clinical features. Int. J. Cancer. 83, 815–822 (1999).
Karellas, M. E., Damjanov, I. & Holzbeierlein, J. M. ITGCN of the testis, contralateral testicular biopsy and bilateral testicular cancer. Urol. Clin. North Am. 34, 119–125 (2007).
Kersemaekers, A. M. et al. Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: an immunohistochemical study for Oct-3/4 and TSPY. Hum. Pathol. 36, 512–521 (2001).
Looijenga, L. H. et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 1, 2244–2250 (2003).
De Jong, J. & Looijenga, L. H. Stem cell marker Oct-3/4 in tumor biology and germ cell tumor diagnostics: history and future. Crit. Rev. Oncog. 12, 171–203 (2006).
Cheng, L. et al. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J. Pathol. 211, 1–9 (2007).
Page, D. C. Hypothesis: a Y-chromosomal gene causes gonadoblastoma in dysgenetic gonads. Development 101, 151–155 (1987).
Li, Y., Vilain, E., Conte, F., Rajpert De Meyts, E. & Lau, Y. F. Testis-specific protein Y-encoded gene is expressed in early and late stages of gonadoblastoma and testicular carcinoma in situ. Urol. Oncol. 25, 141–146 (2007).
Kvist, K., Clasen-Linde, E., Cortes, D., Petersen, B. L. & Thorup, J. Adult immunohistochemical markers fail to detect intratubular germ cell neoplasia in prepubertal boys with cryptorchidism. J. Urol. 191, 1084–1089 (2014).
Hersmus, R. et al. Delayed recognition of disorders of sex development (DSD): a missed opportunity for early diagnosis of malignant germ cell tumors. Int. J. Endocrinol. 2012, 671209 (2012).
Rajpert-De Meyts, E. et al. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS 111, 267–278 (2003).
Jørgensen, N., Giwercman, A., Müller, J. & Skakkebaek, N. E. Immunohistochemical markers of carcinoma in situ of the testis also expressed in normal infantile germ cells. Histopathology 22, 373–378 (1993).
Emerson, R. E. & Cheng, L. Premalignancy of the testis and paratestis. Pathology 45, 264–272 (2013).
Al-Hussain, T., Bakshi, N. & Akhtar, M. Intratubular germ cell neoplasia of the testis: a brief review. Adv. Anat. Pathol. 22, 202–212 (2015).
Hoei-Hansen, C. E. et al. Current approaches for detection of carcinoma in situ testis. Int. J. Androl. 30, 398–404 (2007).
Biermann, K. et al. Diagnostic value of markers M2A, Oct-3/4, AP-2gamma, PLAP and c-KIT in the detection of extragonadal seminomas. Histopathology 49, 290–297 (2006).
Stoop, H. et al. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J. Pathol. 216, 43–54 (2008).
Looijenga, L. H. et al. Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ-cell tumors. Cancer Res. 15, 7674–7678 (2003).
Hersmus, R. et al. New insights into type II germ cell tumor pathogenesis based on the studies of patients with various forms of disorders of sex development (DSD). Mol. Cell Endocrinol. 291, 1–10 (2008).
Pettersson, A., Richiardi, L., Nordenskjold, A., Kaijser, M. & Akre, O. Age at surgery for undescended testis and risk of testicular cancer. N. Engl. J. Med. 356, 1835–1841 (2007).
Martinerie, L., Morel, Y. & Gay, C. L. Impaired puberty, fertility, and final stature in 45,X/46,XY mixed gonadal dysgenetic patients raised as boys. Eur. J. Endocrinol. 166, 687–694 (2012).
Gourlay, W. A., Johnson, H. W. & Pantzar, J. T. Gonadal tumors in disorders of sexual differentiation. Urology 43, 537–540 (1994).
Cools, M., Drop, S. L. S., Wolffenbuttel, K. P., Oosterhuis, J. W. & Looijenga, L. H. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr. Rev. 27, 468–484 (2006).
Hughes, I. A. et al. Consensus statement on management of intersex disorders. Arch. Dis. Child. 91, 554–563 (2006).
Alvarez, N., Lee, T. & Solorzano, C. Complete androgen insensitivity syndrome: the role of the endocrine surgeon. Am. Surg. 71, 241–243 (2005).
Purves, J. T., Miles-Thomas, J., Migeon, C. & Gearhart, J. P. Complete androgen insensitivity: the role of the surgeon. J. Urol. 180, 1716–1719 (2008).
Ahmed, S. F. et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J. Clin. Endocrinol. Metab. 85, 658–665 (2000).
Soule, S. et al. Osteopenia as a feature of the androgen insensitivity syndrome. Clin. Endocrinol. (Oxf.) 43, 671–675 (1995).
Cheikhelard, A. et al. Long-term followup and comparison between genotype and phenotype in 29 cases of complete androgen insensitivity syndrom. J. Urol. 180, 1496–1501 (2008).
Deans, R. et al. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin. Endocrinol. 76, 894–898 (2012).
Hannema, S. E. et al. Testicular development in the complete androgen insensitivity syndrome. J. Pathol. 208, 518–527 (2006).
Hughes, I. A. et al. Androgen insensitivity syndrome. Lancet 380, 419–428 (2012).
Kaprova-Pleskacova, J. et al. Complete androgen insensitivity syndrome: factors influencing gonadal histology including germ cell pathology. Mod. Pathol. 27, 721–730 (2014).
Barthold, J. S. & Gonzales, R. The epidemiology of congenital cryptorchidism, testicular ascent and orchidopexy. J. Urol. 170, 2396–2401 (2003).
Cortes, D., Thorup, J. M. & Visfeldt, J. Cryptorchidism: aspects of fertility and neoplasms. A study including data of 1335 consecutive boys who underwent testicular biopsy simultaneously with surgery for cryptorchidism. Horm. Res. 55, 21–27 (2001).
Skakkebaek, N. E. Testicular dysgenesis syndrome: new epidemiological evidence. Int. J. Androl. 27, 189–191 (2004).
Oosterhuis, J. W. et al. A pathologist's view on the testis biopsy. Int. J. Androl. 34, 14–20 (2011).
Cooper, M. L. et al. Testicular microlithiasis in children and associated testicular cancer. Radiology 270, 857–863 (2014).
Cortes, D., Thorup, J. & Visfeldt, J. Multinucleated spermatogonia in cryptorchid boys: a possible association with an increased risk of testicular malignancy later in life? APMIS 111, 25–30 (2003).
DeCastro, B. J., Peterson, A. C. & Costabile, R. A. A 5-year followup study of asymptomatic men with testicular microlithiasis. J. Urol. 179, 1420–1423 (2008).
Costabile, R. A. How worrisome is testicular microlithiasis? Curr. Opin. Urol. 17, 419–423 (2007).
Peterson, A. C., Bauman, J. M., Light, D. E., McMann, L. P. & Costabile, R. A. The prevalence of testicular microlithiasis in an asymptomatic population of men 18 to 35 years old. J. Urol. 166, 2061–2064 (2001).
Suominen, J. S., Jawaid, W. B. & Losty, P. D. Testicular microlithiasis and associated testicular malignancies in childhood: a systematic review. Pediatr. Blood Cancer 62, 385–388 (2015).
Ritzén, E. M. et al. Nordic consensus on treatment of undescended testes. Acta Paediatr. 96, 638–643 (2007).
Chan, E., Wayne, C. & Nasr, A. Ideal timing of orchiopexy: a systematic review. Pediatr. Surg. Int. 30, 87–97 (2014).
Hadziselimovic, F. & Hoecht, B. Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin. Padiatr. 220, 302–307 (2008).
Kraft, K. H., Canning, D. A., Snyder, H. M. 3rd, Kolon, T. F. Undescended testis histology correlation with adult hormone levels and semen analysis. J. Urol. 188 (Suppl.), 1429–1435 (2012).
Lee, P. A., O'Leary, L. A. & Songer, N. J. Paternity after unilateral cryptorchidism: a controlled study. Pediatrics 98, 676–679 (1996).
Nistal, M., Paniagua, R., Riestra, M. L., Reyes-Múgica, M. & Cajaiba, M. M. Bilateral prepubertal testicular biopsies predict significance of cryptorchidism-associated mixed testicular atrophy, and allow assessment of fertility. Am. J. Surg. Pathol. 31, 1269–1276 (2007).
Thorup, J., Petersen, B. L., Kvist, K. & Cortes, D. Bilateral undescended testes classified according to preoperative and postoperative status of gonadotropins and inhibin B in relation to testicular histopathology at bilateral orchiopexy in infant boys. J. Urol. 188, 1436–1442 (2012).
Hadziselimovic, F., Hadziselimovic, N. O., Demougin, P., Krey, G. & Oakeley, E. Piwi-pathway alteration induces LINE-1 transposon derepression and infertility development in cryptorchidism. Sex. Dev. 9, 98–104 (2015).
Cortes, D., Thorup, J. M. & Beck, B. L. Quantitative histology of germ cells in the undescended testes of human foetuses, neonates and infants. J. Urol. 154, 1188–1192 (1995).
Kenney, L. B. et al. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children's Oncology Group. J. Clin. Oncol. 20, 3408–3416 (2012).
Tournaye, H. et al. Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum. Reprod. Update 10, 525–532 (2004).
Wallace, W. H., Smith, A. G., Kelsey, T. W., Edgar, A. E. & Anderson, R. A. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 15, 1129–1136 (2014).
Hou, M., Andersson, M., Eksborg, S., Soder, O. & Jahnukainen, K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum. Reprod. 22, 1899–1906 (2007).
Nagano, M., Patrizio, P. & Brinster, R. L. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 78, 1225–1233 (2002).
Oatley, J. M., de Avila, D. M., Mc Lean, D. J., Griswold, M. D. & Reeves, J. J. Transplantation of bovine germinal cells into mouse testes. J. Anim. Sci. 80, 1925–1931 (2002).
Choi, Y. J. et al. Long-term follow-up of porcine male germ cells transplanted into mouse testes. Zygote 15, 325–335 (2007).
Zimmermann, S. et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 13, 681–691 (1999).
Hutson, J. M., Li, R., Southwell, B. R., Newgreen, D. & Cousinery, M. Regulation of testicular descent. Pediatr. Surg. Int. 31, 317–325 (2015).
Huston, J. M. A biphasic model for the hormonal control of testicular descent. Lancet 2, 419–421 (1985).
Müller, J. & Skakkebaek, N. E. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int. J. Androl. 6, 143–156 (1983).
Bidlingmaier, F. & Hilscher, W. in Reproductive Biology and Medicine (eds Holstein, A. F. et al.) 34–43 (Diesbach Verlag, 1989).
Cassorla, F. G. et al. Testicular volume during early infancy. J. Pediatr. 99, 742–743 (1981).
Guibourdenche, J. et al. Anti-Müllerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol. Cell. Endocrinol. 211, 55–63 (2003).
De la balze, F. A. et al. Puberal maturation of the normal human testis. A histologic study. J. Clin. Endocrinol. Metab. 20, 266–285 (1960).
Hiort, O. Androgens and puberty. Best Pract. Res. Clin. Endocrinol. Metab. 16, 31–41 (2002).
Diamond, D. A. et al. Comparative assessment of pediatric testicular volume: orchidometer versus ultrasound. J. Urol. 164, 1111–1114 (2000).
Hadziselimovic´, F. Cryptorchidism. Ultrastructure of normal and cryptorchid testis development. Adv. Anat. Embryol. Cell Biol. 53, 3–71 (1977).
Fawcett, D. W., Leak, L. V. & Heidger, P. M. Electron microscopic observations on the structural components of the blood–testis barrier. J. Reprod. Fertil. Suppl. 10, 105–122 (1970).
Hayashi, H. & Harrison, R. G. The development of the interstitial tissue of the human testis. Fertil. Steril. 22, 351–355 (1971).
Acknowledgements
The authors sincerely thank D. MacGregor for his support in the redaction of this manuscript regarding his expertise in testicular histology. A.F. acknowledges the support of grants from the French Society of Pediatric Surgery (SFCP) and the Association for the Development of Biological and Medical Research (ADEREM).
Author information
Authors and Affiliations
Contributions
A.F. researched data for the article, A.F., J.T., J.H. and Y.H. provided a substantial contribution to the discussion of content, A.F. and Y.H. wrote the article, and all authors were involved in the review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Faure, A., Bouty, A., O'Brien, M. et al. Testicular biopsy in prepubertal boys: a worthwhile minor surgical procedure?. Nat Rev Urol 13, 141–150 (2016). https://doi.org/10.1038/nrurol.2015.312
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2015.312
This article is cited by
-
Diagnostic yield and safety of ultrasound-guided percutaneous testicular biopsies in children
Pediatric Radiology (2023)
-
Multidisciplinary consensus on the criteria for fertility preservation in cancer patients
Clinical and Translational Oncology (2022)
-
A Move to Conservativism in Pediatric Urology
Current Pediatrics Reports (2017)