Abstract
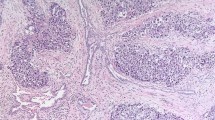
The sophistication and accessibility of modern-day imaging result in frequent detection of small or equivocal lesions of the testes. Traditionally, diagnosis of a testicular lesion with any possibility of malignancy would usually prompt radical orchidectomy. However, awareness is growing that a substantial proportion of these lesions might be benign and that universal application of radical orchidectomy risks frequent overtreatment. Given the potentially profound effects of radical orchidectomy on fertility, endocrine function and psychosexual well-being, particularly in scenarios of an abnormal contralateral testis or bilateral lesions, organ-preserving strategies for equivocal lesions should be considered. Image-based active surveillance can be applied for indeterminate lesions measuring ≤15 mm with a low conversion rate to surgical treatment. However, these outcomes are early and from relatively small, selected cohorts, and concerns prevail regarding the metastatic potential of even small undiagnosed germ cell tumours. No consensus exists on optimal surveillance (short interval (<3 months) ultrasonography is generally adopted); histological sampling is a widespread alternative, involving inguinal delivery of the testis and excisional biopsy of the lesion, with preoperative marking or intraoperative ultrasonographic localization when necessary. Frozen section analysis in this context demonstrates excellent diagnostic accuracy. Histological results support that approximately two-thirds of marker-negative indeterminate solitary testicular lesions measuring ≤25 mm overall are benign. In summary, modern imaging detects many small indeterminate testicular lesions, of which the majority are benign. Awareness is growing of surveillance and organ-sparing diagnostic and treatment strategies with the aim of minimizing rates of overtreatment with radical orchidectomy.
Key points
-
Current data suggest that approximately two-thirds of solitary, clinically indeterminate lesions of the testes measuring ≤25 mm are benign.
-
Urologists are beginning to challenge the traditional approach of recommending radical orchidectomy to men with equivocal lesions.
-
Alternative options to radical orchidectomy include active radiological surveillance (limited outcome data currently available) and excisional biopsy of the lesion to confirm the histopathological diagnosis.
-
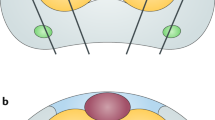
Excisional biopsy is performed via inguinal delivery of the testis, with localization assisted by preoperative needle marking or intraoperative ultrasonography, with or without clamping of the spermatic cord.
-
Excisional biopsy is generally complemented by on-table frozen section analysis, which facilitates proceeding to radical orchidectomy in patients who have appropriately consented, if a germ cell tumour is identified.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wagner, J. & Aron, D. C. Incidentalomas: a “disease” of modern imaging technology. Best Pract. Res. Clin. Endocrinol. Metab. 26, 3–8 (2012).
Corrie, D., Mueller, E. J. & Thompson, I. M. Management of ultrasonically detected nonpalpable testis masses. Urology 38, 429–431 (1991).
Horstman, W. G., Haluszka, M. M. & Burkhard, T. K. Management of testicular masses incidentally discovered by ultrasound. J. Urol. 151, 1263–1265 (1994).
Spaziani, M. et al. The role of scrotal ultrasonography from infancy to puberty. Andrology 9, 1306–1321 (2021).
Bertolotto, M., Sidhu, P. S. & Derchi, L. E. Re: Incidentally detected testicular lesions <10 mm in diameter: can orchidectomy be avoided? BJU Int. https://www.bjuinternational.com/letters/re-incidentally-detected-testicular-lesions/ (2019).
Dieckmann, K. P. et al. Testicular neoplasms: primary tumour size is closely interrelated with histology, clinical staging, and tumour marker expression rates — a comprehensive statistical analysis. Cancers 14, 5447 (2022).
Stephenson, A. et al. Diagnosis and treatment of early stage testicular cancer: AUA guideline. J. Urol. 202, 272–281 (2019).
Shilo, Y. et al. The predominance of benign histology in small testicular masses. Urol. Oncol. 30, 719–722 (2012).
Keske, M. et al. Is testis-sparing surgery safe in small testicular masses? Results of a multicentre study. Can. Urol. Assoc. J. 11, E100–E104 (2017).
Wardak, S. et al. Management of small testicular masses: outcomes from a single-centre specialist multidisciplinary team. BJU Int. 131, 73–81 (2023).
Emmanuel, A. et al. Expedited radical orchidectomy for testicular cancer: compromising fertility outcomes without oncological benefit. Eur. Urol. 80, 766–767 (2021).
Zuniga, A., Lawrentschuk, N. & Jewett, M. A. S. Organ-sparing approaches for testicular masses. Nat. Rev. Urol. 7, 454–464 (2010).
Hayward, R. VOMIT (victims of modern imaging technology) – an acronym for our times. Br. Med. J. 326, 1273 (2003).
Rocher, L. et al. Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur. Radiol. 26, 2268–2278 (2016).
Laguna, M., Albers, P., Algaba, F. & Bokemeyer, C. EAU guidelines on testicular cancer. EAU https://uroweb.org/guidelines/testicular-cancers (2022).
Benelli, A. et al. Evaluation of the decision-making process in the conservative approach to small testicular masses. Urologia 84, 83–87 (2017).
Ates, F. et al. Testis-sparing surgery in small testicular masses not suspected to be malignant. Clin. Genitourin. Cancer 14, e49–e53 (2016).
Bertolotto, M. et al. Imaging of bilateral synchronous testicular tumors of different histologic types and implications for surgical management. J. Ultrasound Med. 35, 2511–2516 (2016).
Carmignani, L. et al. High incidence of benign testicular neoplasms diagnosed by ultrasound. J. Urol. 170, 1783–1786 (2003).
Dell’Atti, L., Fulvi, P. & Benedetto Galosi, A. Are ultrasonographic measurements a reliable parameter to choose non-palpable testicular masses amenable to treatment with sparing surgery? J. BUON 23, 439–443 (2018).
Ferretti, L. et al. Testicular-sparing surgery for bilateral or monorchide testicular tumours: a multicenter study of long-term oncological and functional results. BJU Int. 114, 860–864 (2014).
Gentile, G. et al. Can testis-sparing surgery for small testicular masses be considered a valid alternative to radical orchiectomy? A prospective single-center study. Clin. Genitourin. Cancer 11, 522–526 (2013).
Steiner, H. et al. Frozen section analysis-guided organ-sparing approach in testicular tumors: technique, feasibility, and long-term results. Urology 62, 508–513 (2003).
Connolly, S. S. et al. Carefully selected intratesticular lesions can be safely managed with serial ultrasonography. BJU Int. 98, 1005–1007 (2006).
Avci, A., Erol, B., Eken, C. & Ozgok, Y. Nine cases of nonpalpable testicular mass: an incidental finding in a large scale ultrasonography survey. Int. J. Urol. 15, 833–836 (2008).
Ayati, M. et al. Management of nonpalpable incidental testicular masses: experience with 10 cases. Urol. J. 11, 1892–1895 (2014).
Bieniek, J. M. et al. Prevalence and management of incidental small testicular masses discovered on ultrasonographic evaluation of male infertility. J. Urol. 199, 481–486 (2018).
Fabiani, A. et al. Diagnostic ultrasound-guided excisional testicular biopsy for small (<1 cm) incidental nodules: a single institution experience. Arch. Ital. Urol. Androl. 86, 373–377 (2014).
Leroy, X., Rigot, J. M., Aubert, S., Ballereau, C. & Gosselin, B. Value of frozen section examination for the management of nonpalpable incidental testicular tumors. Eur. Urol. 44, 458–460 (2003).
Manganaro, L. et al. Dynamic contrast-enhanced and diffusion-weighted MR imaging in the characterisation of small, non-palpable solid testicular tumours. Eur. Radiol. 28, 554–564 (2018).
Powell, T. M. & Tarter, T. H. Management of nonpalpable incidental testicular masses. J. Urol. 176, 96–99 (2006).
Pozza, C. et al. Diagnostic value of qualitative and strain ratio elastography in the differential diagnosis of non-palpable testicular lesions. Andrology 4, 1193–1203 (2016).
Sbrollini, G. et al. Diagnostic-therapeutic pathway for small lesions of the testis. Arch. Ital. Urol. Androl. 86, 397–399 (2014).
Assaf, G. J. Non-palpable testicular lesion: the case for testicular preservation. Can. J. Urol. 13, 3034–3038 (2006).
Hallak, J. et al. Organ-sparing microsurgical resection of incidental testicular tumors plus microdissection for sperm extraction and cryopreservation in azoospermic patients: surgical aspects and technical refinements. Urology 73, 887–891 (2009).
Rolle, L. et al. Microsurgical “testis-sparing” surgery for nonpalpable hypoechoic testicular lesions. Urology 68, 381–385 (2006).
La Vecchia, C. et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann. Oncol. 21, 1323–1360 (2010).
Hindley, R. G., Chandra, A., Saunders, A. & O’Brien, T. S. Impalpable testis cancer. BJU Int. 92, 572–574 (2003).
Del Real, O. J., Calvo de la Barra, C. I., Jiménez, J. A., Sepulveda, F. & Domínguez, J. Predicting malignancy in small testicular lesions. Cent. Eur. J. Urol. 75, 47–51 (2022).
Müller, T. et al. Management of incidental impalpable intratesticular masses of ≤5 mm in diameter. BJU Int. 98, 1001–1004 (2006).
Li, Q., Vij, A., Hahn, P. F., Xiang, F. & Samir, A. E. The value of active ultrasound surveillance for patients with small testicular lesions. Ultrasound Q. 33, 23–27 (2017).
Passarella, M., Usta, M. F., Bivalacqua, T. J., Hellstrom, W. J. & Davis, R. Testicular-sparing surgery: a reasonable option in selected patients with testicular lesions. BJU Int. 91, 337–340 (2003).
Bojanic, N. et al. Testis sparing surgery for treatment of small testicular lesions: is it feasible even in germ cell tumors? J. Surg. Oncol. 115, 287–290 (2017).
Colpi, G. M. et al. Testicular-sparing microsurgery for suspected testicular masses. BJU Int. 96, 67–69 (2005).
Dell’Atti, L. Efficacy of ultrasound-guided testicle-sparing surgery for small testicular masses. J. Ultrasound 19, 29–33 (2016).
Gentile, G. et al. Testis sparing surgery of small testicular masses: retrospective analysis of a multicenter cohort. J. Urol. 203, 760–766 (2020).
Khan, M. J., Bedi, N., Rahimi, M. N. C. & Kalsi, J. Testis sparing surgery for small testicular masses and frozen section assessment. Cent. Eur. J. Urol. 71, 304–309 (2018).
Lagabrielle, S. et al. Testicular tumours discovered during infertility workup are predominantly benign and could initially be managed by sparing surgery. J. Surg. Oncol. 118, 630–635 (2018).
Staudacher, N. et al. Organ-sparing surgery in testicular tumor: is this the right approach for lesions ≤20 mm? J. Clin. Med. 9, 2911 (2020).
Tuygun, C. et al. Evaluation of frozen section results in patients who have suspected testicular masses: a preliminary report. Urol. J. 11, 1253–1257 (2014).
De Stefani, S. et al. Microsurgical testis-sparing surgery in small testicular masses: seven years retrospective management and results. Urology 79, 858–862 (2012).
Lewicki, A. et al. Incidental findings and how to manage them: testis–A WFUMB position paper. Ultrasound Med. Biol. 47, 2787–2802 (2021).
Capelouto, C. C., Clark, P. E., Ransil, B. J. & Loughlin, K. R. A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? J. Urol. 153, 981–985 (1995).
Pratsinis, M. et al. Metastatic potential of small testicular germ cell tumors: implications for surveillance of small testicular masses. Eur. Urol. Open Sci. 40, 16–18 (2022).
Comiter, C. V. et al. Nonpalpable intratesticular masses detected sonographically. J. Urol. 154, 1367–1369 (1995).
Luzurier, A. et al. Qualitative and quantitative contrast-enhanced ultrasonography for the characterisation of non-palpable testicular tumours. Clin. Radiol. 73, 322.e1–322.e9 (2018).
Scandura, G. et al. Incidentally detected testicular lesions <10 mm in diameter: can orchidectomy be avoided? BJU Int. 121, 575–582 (2018).
Schwen, Z. R. et al. Testicular ultrasound underestimates the size of small testicular masses: a radiologic–pathologic correlation study. World J. Urol. 39, 3399–3405 (2021).
Pedersen, M. R., Osther, P. J., Soerensen, F. B. & Rafaelsen, S. R. Testicular microlithiasis: patient compliance in a two-year follow-up program. Ultrasound Int. Open 2, E113–E116 (2016).
Goldstein, M. & Waterhouse, K. When to use the Chevassu maneuver during exploration of intrascrotal masses. J. Urol. 130, 1199–1200 (1983).
Eifler, J. B.Jr, King, P. & Schlegel, P. N. Incidental testicular lesions found during infertility evaluation are usually benign and may be managed conservatively. J. Urol. 180, 261–265 (2008).
Hopps, C. V. & Goldstein, M. Ultrasound guided needle localization and microsurgical exploration for incidental nonpalpable testicular tumors. J. Urol. 168, 1084–1087 (2002).
Galosi, A. B. et al. Testicular sparing surgery in small testis masses: a multinstitutional experience. Arch. Ital. Urol. Androl. 88, 320–324 (2016).
Brunocilla, E. et al. Testis-sparing surgery for the conservative management of small testicular masses: an update. Anticancer Res. 33, 5205–5210 (2013).
Soh, E., Berman, L. H., Grant, J. W., Bullock, N. & Williams, M. V. Ultrasound-guided core-needle biopsy of the testis for focal indeterminate intratesticular lesions. Eur. Radiol. 18, 2990–2996 (2008).
Nason, G. J. et al. Partial orchiectomy: the Princess Margaret Cancer Centre experience. Urol. Oncol. 38, 605.e19–605.e24 (2020).
Gobbo, A. et al. Is testis sparing surgery safe in patients with incidental small testicular lesions referring to a fertility center? A retrospective analysis reporting factors correlated to malignancy and long-term oncological outcomes. Urol. Oncol. 40, 457.e9–457.e16 (2022).
Paffenholz, P., Held, L., Loosen, S. H., Pfister, D. & Heidenreich, A. Testis sparing surgery for benign testicular masses: diagnostics and therapeutic approaches. J. Urol. 200, 353–360 (2018).
Drudi, F. M. et al. Detection of small testicular masses in monorchid patients using US, CPDUS, CEUS and US-guided biopsy. J. Ultrasound 19, 25–28 (2016).
Lock, G. et al. Contrast-enhanced ultrasound and real-time elastography for the diagnosis of benign Leydig cell tumors of the testis — a single center report on 13 cases. Ultraschall Med. 35, 534–539 (2014).
Huang, D. Y. & Sidhu, P. S. Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br. J. Radiol. 85, S41–S53 (2012).
Isidori, A. M. et al. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology 273, 606–618 (2014).
Khanna, M. et al. Diagnostic performance of multi-parametric MRI to differentiate benign sex cord stromal tumors from malignant (non-stromal and stromal) testicular neoplasms. Abdom. Radiol. 46, 319–330 (2021).
Manganaro, L. et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur. Radiol. 25, 3586–3595 (2015).
Tsili, A. C. et al. When to ask for an MRI of the scrotum. Andrology 9, 1395–1409 (2021).
Tsili, A. C. et al. MRI of the scrotum: recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur. Radiol. 28, 31–43 (2018).
Goddi, A., Sacchi, A., Magistretti, G., Almolla, J. & Salvadore, M. Real-time tissue elastography for testicular lesion assessment. Eur. Radiol. 22, 721–730 (2012).
Auer, T. et al. Value of multiparametric US in the assessment of intratesticular lesions. Radiology 285, 640–649 (2017).
Feliciani, G. et al. The potential role of MR based radiomic biomarkers in the characterization of focal testicular lesions. Sci. Rep. 11, 3456 (2021).
Milose, J. C., Filson, C. P., Weizer, A. Z., Hafez, K. S. & Montgomery, J. S. Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open Access. J. Urol. 4, 1–8 (2011).
Dieckmann, K. P. et al. Serum levels of microRNA-371a-3p (M371 test) as a new biomarker of testicular germ cell tumors: results of a prospective multicentric study. J. Clin. Oncol. 37, 1412–1423 (2019).
Leao, R. et al. Circulating microRNAs, the next-generation serum biomarkers in testicular germ cell tumours: a systematic review. Eur. Urol. 80, 456–466 (2021).
Hotaling, J. M. & Walsh, T. J. Male infertility: a risk factor for testicular cancer. Nat. Rev. Urol. 6, 550–556 (2009).
Toren, P. J. et al. Small incidentally discovered testicular masses in infertile men – is active surveillance the new standard of care? J. Urol. 183, 1373–1377 (2010).
Raman, J. D., Nobert, C. F. & Goldstein, M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J. Urol. 174, 1819–1822 (2005).
Bertolotto, M. et al. Contrast-enhanced ultrasound for characterizing renal masses. Eur. J. Radiol. 105, 41–48 (2018).
Cicero, C. et al. Multiple, synchronous lesions of differing histology within the same testis: ultrasonographic and pathologic correlations. Urology 121, 125–131 (2018).
Bertolotto, M. et al. Multiparametric US for scrotal diseases. Abdom. Radiol. 43, 899–917 (2018).
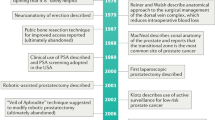
Sathianathen, N. J., Konety, B. R., Crook, J., Saad, F. & Lawrentschuk, N. Landmarks in prostate cancer. Nat. Rev. Urol. 15, 627–642 (2018).
Mir, M. C. et al. Role of active surveillance for localized small renal masses. Eur. Urol. Oncol. 1, 177–187 (2018).
Stoll, S. et al. Incidental detection of impalpable testicular neoplasm by sonography. AJR Am. J. Roentgenol. 146, 349–350 (1986).
Browne, R. F. et al. Technical report. Intra-operative ultrasound-guided needle localization for impalpable testicular lesions. Clin. Radiol. 58, 566–569 (2003).
Heidenreich, A. & Angerer-Shpilenya, M. Organ-preserving surgery for testicular tumours. BJU Int. 109, 474–490 (2012).
Matei, D. V. et al. Reliability of frozen section examination in a large cohort of testicular masses: what did we learn? Clin. Genitourin. Cancer 15, e689–e696 (2017).
Elert, A. et al. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur. Urol. 41, 290–293 (2002).
Tokuc, R., Sakr, W., Pontes, J. E. & Haas, G. P. Accuracy of frozen section examination of testicular tumors. Urology 40, 512–516 (1992).
Ehrlich, Y., Konichezky, M., Yossepowitch, O. & Baniel, J. Multifocality in testicular germ cell tumors. J. Urol. 181, 1114–1120 (2009).
Huyghe, E. et al. Conservative management of small testicular tumors relative to carcinoma in situ prevalence. J. Urol. 173, 820–823 (2005).
Drudi, F. M. et al. CEUS time intensity curves in the differentiation between Leydig cell carcinoma and seminoma: a multicenter study. Ultraschall Med. 3, 201–205 (2016).
Author information
Authors and Affiliations
Contributions
S.M.C., J.W.M. and P.R. researched data for the article. S.M.C., A.McG., I.M.C. and N.F.D. contributed substantially to discussion of the content. S.M.C. wrote the article. S.M.C., P.R., C.B., A.McG. I.M.C. and N.F.D. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Dean Huangand the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A search of MEDLINE, Scopus and Cochrane databases was performed for full-text, English-language articles published during the period 1990–2022. The search strategy included search terms: (“Indeterminate” OR “uncertain” OR “equivocal” OR “incidental” OR “small” OR “suspected” OR “suspicious”) AND (“testis” (MeSH term) OR “testicular”) AND (“lesion” OR “mass” OR “neoplasm” OR “abnormality”). Titles and abstracts of potentially eligible publications were screened, and full texts of potentially relevant articles were retrieved. A reference trawl of included articles was also performed. Studies reporting upon the diagnostic evaluation and/or surveillance of indeterminate lesions of the testes were considered eligible for inclusion. Randomized trials and observational studies, including non-comparative case series, were included. Review articles, conference abstracts and case reports were excluded. Lesions radiologically measuring ≤25 mm in maximum dimension and considered indeterminate were included. Lesions were considered indeterminate if reported as such by the study authors for any reason, including small size making characterization difficult or demonstration of radiological features atypical of malignancy. Both lesions existing in isolation and those discovered in the presence of additional equivocal or suspicious abnormalities were included. The size threshold of ≤25 mm was decided upon following a scoping review of the literature, which identified an upper size limit of 21–25 mm in a number of high-quality studies16,17,18,19,20,21,22,23. Studies reporting on lesions existing in the presence of known metastases, with clinical presumption of a diagnosis of germ cell tumour or with a definitive benign nature were excluded. Studies including lesions measuring >25 mm in one plane were excluded, except where it was possible to extrapolate complete data on individual lesions meeting the inclusion criteria. A quality assessment of each included study was conducted by two reviewers, with the Newcastle Ottawa Scale (NOS) for non-randomized studies applied. Relevant variables were retrieved from all studies, using template-based data extraction. Data were compiled with the results synthesized and presented in narrative format in this Review.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Croghan, S.M., Malak, J.W., Rohan, P. et al. Diagnosis and management of indeterminate testicular lesions. Nat Rev Urol 21, 7–21 (2024). https://doi.org/10.1038/s41585-023-00786-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00786-3
This article is cited by
-
Ultrasound-based deep learning radiomics nomogram for risk stratification of testicular masses: a two-center study
Journal of Cancer Research and Clinical Oncology (2025)