Abstract
Carcinoma of the urinary bladder is a common malignancy and a major cause of morbidity and mortality in the western world. Current understanding of etiology, disease process, molecular characteristics and management principles make urothelial carcinoma an ideal candidate for screening. The capacity of traditional noninvasive diagnostic procedures such as microhematuria testing and urine cytology to be used as stand-alone screening techniques is limited, however. New qualitative and quantitative molecular screening modalities can detect cellular and subcellular alterations that are often exclusively associated with urothelial carcinoma. Such alterations can be detected in a noninvasive manner, using urine as a marker source, with reasonable sensitivity and specificity. Application of several molecular assays in conjunction with traditional screening methods has had promising results. We propose an evidence-based and risk-based approach to future bladder cancer screening. Such an approach would harness the reasonable sensitivity, ease of use and cost-effectiveness of microhematuria testing, plus the specificity of molecular tests, to target high-risk populations for screening. The ultimate goals are to identify susceptible individuals, detect bladder tumors before they invade using unobtrusive and cost-effective methods, and optimize surveillance strategies for long-term follow-up.
Key Points
-
The incidence of bladder cancer is on the rise, and mortality rates and overall prognosis have not changed dramatically for three decades, despite improved management
-
Detection of microhematuria could be a useful screening tool, but its specificity for bladder cancer is poor
-
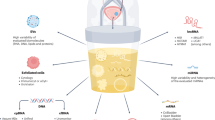
Newer tests use urine as the source of markers of molecular change in, or altered products released from, bladder tumor cells
-
Some molecular tests are qualitative point-of-care assays, and some require specialized laboratory facilities for quantitative determination of marker alterations, while others enhance the quality of information gleaned from routine urine cytology
-
Using a combination of molecular tests, with or without cytology, could reduce the frequency of cystoscopy
-
Future bladder cancer screening algorithms will apply traditional and molecular tests in a stepwise fashion on the basis of an individual patient's risk of disease development
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 (2009).
Botteman, M. F., Pashos, C. L., Redaelli, A., Laskin, B. & Hauser, R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 21, 1315–1330 (2003).
Mitra, A. P., Datar, R. H. & Cote, R. J. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J. Clin. Oncol. 24, 5552–5564 (2006).
Mitra, A. P. & Cote, R. J. Molecular pathogenesis and diagnostics of bladder cancer. Annu. Rev. Pathol. 4, 251–285 (2009).
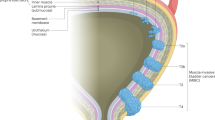
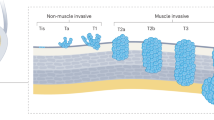
Bryan, R. T. & Wallace, D. M. 'Superficial' bladder cancer—time to uncouple pT1 tumours from pTa tumours. BJU Int. 90, 846–852 (2002).
Pasin, E., Josephson, D. Y., Mitra, A. P., Cote, R. J. & Stein, J. P. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev. Urol. 10, 31–43 (2008).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Foresman, W. H. & Messing, E. M. Bladder cancer: natural history, tumor markers, and early detection strategies. Semin. Surg. Oncol. 13, 299–306 (1997).
Parekh, D. J., Bochner, B. H. & Dalbagni, G. Superficial and muscle-invasive bladder cancer: principles of management for outcomes assessments. J. Clin. Oncol. 24, 5519–5527 (2006).
Wakui, M. & Shiigai, T. Urinary tract cancer screening through analysis of urinary red blood cell volume distribution. Int. J. Urol. 7, 248–253 (2000).
Grossfeld, G. D. et al. Asymptomatic microscopic hematuria in adults: summary of the AUA best practice policy recommendations. Am. Fam. Physician 63, 1145–1154 (2001).
Messing, E. M., Young, T. B., Hunt, V. B., Wehbie, J. M. & Rust, P. Urinary tract cancers found by homescreening with hematuria dipsticks in healthy men over 50 years of age. Cancer 64, 2361–2367 (1989).
Messing, E. M. et al. Hematuria home screening: repeat testing results. J. Urol. 154, 57–61 (1995).
Messing, E. M. et al. Home screening for hematuria: Results of a multiclinic study. J. Urol. 148, 289–292 (1992).
Schroeder, G. L. et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J. Urol. 172, 1123–1126 (2004).
Ramakumar, S. et al. Comparison of screening methods in the detection of bladder cancer. J. Urol. 161, 388–394 (1999).
Halling, K. C. et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J. Urol. 167, 2001–2006 (2002).
Britton, J. P., Dowell, A. C., Whelan, P. & Harris, C. M. A community study of bladder cancer screening by the detection of occult urinary bleeding. J. Urol. 148, 788–790 (1992).
Messing, E. M. et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology 45, 387–396 (1995).
Carmack, A. J. & Soloway, M. S. The diagnosis and staging of bladder cancer: from RBCs to TURs. Urology 67, 3–8 (2006).
Lotan, Y. & Roehrborn, C. G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology 61, 109–118 (2003).
Wiener, H. G., Vooijs, G. P. & van't Hof-Grootenboer, B. Accuracy of urinary cytology in the diagnosis of primary and recurrent bladder cancer. Acta Cytol. 37, 163–169 (1993).
Mitra, A. P., Birkhahn, M., Penson, D. F. & Cote, R. J. Urine biomarkers for the detection of urothelial cancer [online], (2009).
Cheng, Z. Z. et al. Complement factor H as a marker for detection of bladder cancer. Clin. Chem. 51, 856–863 (2005).
Black, P. C., Brown, G. A. & Dinney, C. P. Molecular markers of urothelial cancer and their use in the monitoring of superficial urothelial cancer. J. Clin. Oncol. 24, 5528–5535 (2006).
Abd El Gawad, I. A. et al. Comparative study of NMP-22, telomerase, and BTA in the detection of bladder cancer. J. Egypt. Natl Canc. Inst. 17, 193–202 (2005).
Eissa, S. et al. Comparative evaluation of the nuclear matrix protein, fibronectin, urinary bladder cancer antigen and voided urine cytology in the detection of bladder tumors. J. Urol. 168, 465–469 (2002).
Miyanaga, N. et al. Usefulness of urinary NMP22 to detect tumor recurrence of superficial bladder cancer after transurethral resection. Int. J. Clin. Oncol. 8, 369–373 (2003).
Ponsky, L. E. et al. Screening and monitoring for bladder cancer: refining the use of NMP22. J. Urol. 166, 75–78 (2001).
Grossman, H. B. et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA 295, 299–305 (2006).
Lokeshwar, V. B. & Selzer, M. G. Urinary bladder tumor markers. Urol. Oncol. 24, 528–537 (2006).
Lokeshwar, V. B. et al. Urinary hyaluronic acid and hyaluronidase: markers for bladder cancer detection and evaluation of grade. J. Urol. 163, 348–356 (2000).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Kavaler, E., Landman, J., Chang, Y., Droller, M. J. & Liu, B. C. Detecting human bladder carcinoma cells in voided urine samples by assaying for the presence of telomerase activity. Cancer 82, 708–714 (1998).
Schmetter, B. S. et al. A multicenter trial evaluation of the fibrin/fibrinogen degradation products test for detection and monitoring of bladder cancer. J. Urol. 158, 801–805 (1997).
Topsakal, M., Karadeniz, T., Anac, M., Donmezer, S. & Besisik, A. Assessment of fibrin-fibrinogen degradation products (Accu-Dx) test in bladder cancer patients. Eur. Urol. 39, 287–291 (2001).
Mitra, A. P., Datar, R. H. & Cote, R. J. Molecular staging of bladder cancer. BJU Int. 96, 7–12 (2005).
Mitra, A. P., Lin, H., Cote, R. J. & Datar, R. H. Biomarker profiling for cancer diagnosis, prognosis and therapeutic management. Natl Med. J. India 18, 304–312 (2005).
Mitra, A. P., Lin, H., Datar, R. H. & Cote, R. J. Molecular biology of bladder cancer: prognostic and clinical implications. Clin. Genitourin. Cancer 5, 67–77 (2006).
Mitra, A. P., Birkhahn, M. & Cote, R. J. p53 and retinoblastoma pathways in bladder cancer. World J. Urol. 25, 563–571 (2007).
Lokeshwar, V. B. et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology 66, 35–63 (2005).
Halling, K. C. Vysis® UroVysion for the detection of urothelial carcinoma. Expert Rev. Mol. Diagn. 3, 507–519 (2003).
Halling, K. C. et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J. Urol. 164, 1768–1775 (2000).
Bubendorf, L. et al. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am. J. Clin. Pathol. 116, 79–86 (2001).
Sarosdy, M. F. et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J. Urol. 168, 1950–1954 (2002).
Skacel, M. et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J. Urol. 169, 2101–2105 (2003).
Feil, G. et al. Accuracy of the ImmunoCyt assay in the diagnosis of transitional cell carcinoma of the urinary bladder. Anticancer Res. 23, 963–967 (2003).
Hautmann, S. et al. Immunocyt and the HA-HAase urine tests for the detection of bladder cancer: A side-by-side comparison. Eur. Urol. 46, 466–471 (2004).
Têtu, B., Tiguert, R., Harel, F. & Fradet, Y. ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod. Pathol. 18, 83–89 (2005).
Mian, C. et al. The value of the ImmunoCyt/uCyt+ test in the detection and follow-up of carcinoma in situ of the urinary bladder. Anticancer Res. 25, 3641–3644 (2005).
Mian, C. et al. ImmunoCyt: a new tool for detecting transitional cell cancer of the urinary tract. J. Urol. 161, 1486–1489 (1999).
Lodde, M. et al. Detection of upper urinary tract transitional cell carcinoma with ImmunoCyt: a preliminary report. Urology 58, 362–366 (2001).
Pfister, C. et al. ImmunoCyt test improves the diagnostic accuracy of urinary cytology: results of a French multicenter study. J. Urol. 169, 921–924 (2003).
Mian, C. et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer 108, 60–65 (2006).
Lodde, M. et al. uCyt+ test: alternative to cystoscopy for less-invasive follow-up of patients with low risk of urothelial carcinoma. Urology 67, 950–954 (2006).
Birkhahn, M., Mitra, A. P. & Cote, R. J. Molecular markers for bladder cancer: the road to a multimarker approach. Expert Rev. Anticancer Ther. 7, 1717–1727 (2007).
Vriesema, J. L., Poucki, M. H., Kiemeney, L. A. & Witjes, J. A. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: a utility analysis. Urology 56, 793–797 (2000).
Surveillance Epidemiology and End Results Cancer Stat Fact Sheets—Urinary Bladder [online], (2009).
US Department of Health and Human Services Agency for Healthcare Research and Quality Screening for Bladder Cancer in Adults [online], (2004).
Ruttenberg, R. & Powers, M. Economics of notification and medical screening for high-risk workers. J. Occup. Med. 28, 757–764 (1986).
US Department of Labor Consumer Price Index Inflation Calculator [online], (2009).
Lotan, Y., Svatek, R. S. & Sagalowsky, A. I. Should we screen for bladder cancer in a high-risk population?: a cost per life-year saved analysis. Cancer 107, 982–990 (2006).
Mitra, A. P. & Cote, R. J. Searching for novel therapeutics and targets: insights from clinical trials. Urol. Oncol. 25, 341–343 (2007).
Madeb, R., Golijanin, D., Knopf, J. & Messing, E. M. Current state of screening for bladder cancer. Expert Rev. Anticancer Ther. 7, 981–987 (2007).
Madeb, R. & Messing, E. M. Long-term outcome of home dipstick testing for hematuria. World J. Urol. 26, 19–24 (2008).
Lotan, Y. & Shariat, S. F. Impact of risk factors on the performance of the nuclear matrix protein 22 point-of-care test for bladder cancer detection. BJU Int. 101, 1362–1367 (2008).
Lotan, Y. et al. Bladder cancer screening in a high risk asymptomatic population using a point of care urine based protein tumor marker. J. Urol. 182, 52–57 (2009).
Steiner, H. et al. Early results of bladder-cancer screening in a high-risk population of heavy smokers. BJU Int. 102, 291–296 (2008).
Kumar, A., Kumar, R. & Gupta, N. P. Comparison of NMP22 BladderChek test and urine cytology for the detection of recurrent bladder cancer. Jpn J. Clin. Oncol. 36, 172–175 (2006).
Kibar, Y. et al. Prognostic value of cytology, nuclear matrix protein 22 (NMP22) test, and urinary bladder cancer II (UBC II) test in early recurrent transitional cell carcinoma of the bladder. Ann. Clin. Lab. Sci. 36, 31–38 (2006).
Mian, C. et al. Multiprobe fluorescence in situ hybridisation: prognostic perspectives in superficial bladder cancer. J. Clin. Pathol. 59, 984–987 (2006).
May, M. et al. Comparative diagnostic value of urine cytology, UBC-ELISA, and fluorescence in situ hybridization for detection of transitional cell carcinoma of urinary bladder in routine clinical practice. Urology 70, 449–453 (2007).
Bergman, J., Reznichek, R. C. & Rajfer, J. Surveillance of patients with bladder carcinoma using fluorescent in-situ hybridization on bladder washings. BJU Int. 101, 26–29 (2008).
Raitanen, M. P. The role of BTA stat test in follow-up of patients with bladder cancer: results from FinnBladder studies. World J. Urol. 26, 45–50 (2008).
Gacci, M. et al. Pre and postoperative quantitative detection of fragments of cytokeratins 8 and 18 (UBC IRMA) as markers of early recurrence of superficial bladder tumor. Arch. Ital. Urol. Androl. 78, 5–10 (2006).
van der Poel, H. G. et al. Quanticyt: karyometric analysis of bladder washing for patients with superficial bladder cancer. Urology 48, 357–364 (1996).
Zheng, S. et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 1162, 154–161 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Mitra, A., Cote, R. Molecular screening for bladder cancer: progress and potential. Nat Rev Urol 7, 11–20 (2010). https://doi.org/10.1038/nrurol.2009.236
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2009.236
This article is cited by
-
The human TRAM1 locus expresses circular RNAs
Scientific Reports (2021)
-
Landmarks in the treatment of muscle-invasive bladder cancer
Nature Reviews Urology (2017)
-
Three serum metabolite signatures for diagnosing low-grade and high-grade bladder cancer
Scientific Reports (2017)
-
Urinary calprotectin: a new diagnostic marker in urothelial carcinoma of the bladder
World Journal of Urology (2014)
-
Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers
Nature Reviews Urology (2013)