Key Points
-
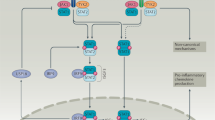
Type I interferon has a pathogenic role in many rheumatic conditions, including systemic lupus erythematosus, Sjögren syndrome, myositis and systemic sclerosis.
-
Many genetic risk factors for rheumatic diseases lie within the type I interferon pathway as gain-of-function polymorphisms, and both polygenic and monogenic influences have been described.
-
Stratifying patients by type I interferon activity levels will inform us about both disease pathogenesis and treatment response in rheumatic diseases.
-
A number of therapeutics that target type I interferons, the type I interferon receptor, or the type I interferon pathway are currently in various stages of development.
Abstract
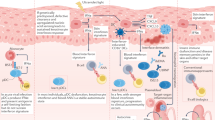
The type I interferon pathway has been implicated in the pathogenesis of a number of rheumatic diseases, including systemic lupus erythematosus, Sjögren syndrome, myositis, systemic sclerosis, and rheumatoid arthritis. In normal immune responses, type I interferons have a critical role in the defence against viruses, yet in many rheumatic diseases, large subgroups of patients demonstrate persistent activation of the type I interferon pathway. Genetic variations in type I interferon-related genes are risk factors for some rheumatic diseases, and can explain some of the heterogeneity in type I interferon responses seen between patients within a given disease. Inappropriate activation of the immune response via Toll-like receptors and other nucleic acid sensors also contributes to the dysregulation of the type I interferon pathway in a number of rheumatic diseases. Theoretically, differences in type I interferon activity between patients might predict response to immune-based therapies, as has been demonstrated for rheumatoid arthritis. A number of type I interferon and type I interferon pathway blocking therapies are currently in clinical trials, the results of which are promising thus far. This Review provides an overview of the many ways in which the type I interferon system affects rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Rice, G. et al. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am. J. Hum. Genet. 81, 713–725 (2007).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Crow, M. K. & Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 5, 279–287 (2003).
Kariuki, S. N. et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-α identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun. 16, 15–23 (2015).
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 258–267 (1957).
Pestka, S., Krause, C. D. & Walter, M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004).
Pestka, S. Purification and cloning of interferon α. Curr. Top. Microbiol. Immunol. 316, 23–37 (2007).
Ito, T., Kanzler, H., Duramad, O., Cao, W. & Liu, Y. J. Specialization, kinetics, and repertoire of type I interferon responses by human plasmacytoid predendritic cells. Blood 107, 2423–2431 (2006).
Prakash, A., Smith, E., Lee, C. K. & Levy, D. E. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 280, 18651–18657 (2005).
Severa, M. et al. Sensitization to TLR7 agonist in IFN-β-preactivated dendritic cells. J. Immunol. 178, 6208–6216 (2007).
Nir, U., Maroteaux, L., Cohen, B. & Mory, I. Priming affects the transcription rate of human interferon-β1 gene. J. Biol. Chem. 260, 14242–14247 (1985).
Weiss, G. et al. MyD88 drives the IFN-β response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J. Immunol. 189, 2860–2868 (2012).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Oliveira, L., Sinicato, N. A., Postal, M., Appenzeller, S. & Niewold, T. B. Dysregulation of antiviral helicase pathways in systemic lupus erythematosus. Front. Genet. 5, 418 (2014).
Crow, Y. J. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Mavragani, C. P. et al. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol. 68, 2686–2696 (2016).
Wang, Y. et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity 46, 393–404 (2017).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Shrivastav, M. & Niewold, T. B. Nucleic acid sensors and type I interferon production in systemic lupus erythematosus. Front. Immunol. 4, 319 (2013).
Blasius, A. L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Jensen, M. A. & Niewold, T. B. Interferon regulatory factors: critical mediators of human lupus. Transl Res. 165, 283–295 (2015).
Yarilina, A., Park-Min, K. H., Antoniv, T., Hu, X. & Ivashkiv, L. B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9, 378–387 (2008).
Venkatesh, D. et al. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-β autocrine signaling to promote monocyte recruitment. Immunity 38, 1025–1037 (2013).
Takayanagi, H. et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416, 744–749 (2002).
Xiong, Q., Zhang, L., Ge, W. & Tang, P. The roles of interferons in osteoclasts and osteoclastogenesis. Joint Bone Spine 83, 276–281 (2016).
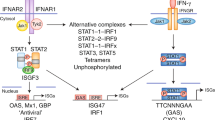
de Weerd, N. A. et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat. Immunol. 14, 901–907 (2013).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Sharma, S. et al. Widely divergent transcriptional patterns between SLE patients of different ancestral backgrounds in sorted immune cell populations. J. Autoimmun. 60, 51–58 (2015).
Goh, K. C., Haque, S. J. & Williams, B. R. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18, 5601–5608 (1999).
Kaur, S. et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl Acad. Sci. USA 105, 4808–4813 (2008).
Fink, K. et al. IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response. Cell Res. 23, 673–690 (2013).
Higgs, B. W. et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 70, 2029–2036 (2011).
Karonitsch, T. et al. Activation of the interferon-gamma signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 60, 1463–1471 (2009).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 1548–1550 (2016).
Becker, A. M. et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS ONE 8, e67003 (2013).
Weckerle, C. E. et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 63, 1044–1053 (2011).
Jabs, W. J., Hennig, C., Zawatzky, R. & Kirchner, H. Failure to detect antiviral activity in serum and plasma of healthy individuals displaying high activity in ELISA for IFN-α and IFN-β. J. Interferon Cytokine Res. 19, 463–469 (1999).
Wilson, D. R. et al. The Simoa HD-I analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Lab. Autom. 21, 533–547 (2016).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010).
Meyer, S. et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell 166, 582–595 (2016).
Ronnblom, L. E., Alm, G. V. & Oberg, K. E. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 227, 207–210 (1990).
Niewold, T. B. & Swedler, W. I. Systemic lupus erythematosus arising during interferon-α therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin. Rheumatol. 24, 178–181 (2005).
Niewold, T. B., Hua, J., Lehman, T. J., Harley, J. B. & Crow, M. K. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 8, 492–502 (2007).
Kariuki, S. N. et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res. Ther. 12, R151 (2010).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Ghodke-Puranik, Y. & Niewold, T. B. Genetics of the type I interferon pathway in systemic lupus erythematosus. Int. J. Clin. Rheumtol. https://doi.org/10.2217/ijr.13.58 (2013).
Kariuki, S. N. et al. Cutting edge: Autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-α in lupus patients in vivo. J. Immunol. 182, 34–38 (2009).
Robinson, T. et al. Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-α and serologic autoimmunity in lupus patients. J. Immunol. 187, 1298–1303 (2011).
Barnes, B. J., Moore, P. A. & Pitha, P. M. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon α genes. J. Biol. Chem. 276, 23382–23390 (2001).
Yasuda, K. et al. Interferon regulatory factor-5 deficiency ameliorates disease severity in the MRL/lpr mouse model of lupus in the absence of a mutation in DOCK2. PLoS ONE 9, e103478 (2014).
Graham, R. R. et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc. Natl Acad. Sci. USA 104, 6758–6763 (2007).
Harley, J. B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Lessard, C. J. et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat. Genet. 45, 1284–1292 (2013).
Radstake, T. R. et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat. Genet. 42, 426–429 (2010).
Dieguez-Gonzalez, R. et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 58, 1264–1274 (2008).
Nordang, G. B. et al. Interferon regulatory factor 5 gene polymorphism confers risk to several rheumatic diseases and correlates with expression of alternative thymic transcripts. Rheumatology (Oxford) 51, 619–626 (2012).
Niewold, T. B. et al. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 58, 2481–2487 (2008).
Niewold, T. B. et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann. Rheum. Dis. 71, 463–468 (2012).
Cherian, T. S. et al. Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus. Arthritis Rheum. 64, 3383–3387 (2012).
Fu, Q. et al. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis Rheum. 63, 749–754 (2011).
Lessard, C. J. et al. Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am. J. Hum. Genet. 90, 648–660 (2012).
Salloum, R. et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-α activity in lupus patients. Arthritis Rheum. 62, 553–561 (2010).
Chrabot, B. S. et al. Genetic variation near IRF8 is associated with serologic and cytokine profiles in systemic lupus erythematosus and multiple sclerosis. Genes Immun. 14, 471–478 (2013).
Pothlichet, J. et al. A loss-of-function variant of the antiviral molecule MAVS is associated with a subset of systemic lupus patients. EMBO Mol. Med. 3, 142–152 (2011).
Kariuki, S. N., Crow, M. K. & Niewold, T. B. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-α activity and low tumor necrosis factor α levels in patients with lupus. Arthritis Rheum. 58, 2818–2823 (2008).
Wang, Y. et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type I interferon-dependent immunity. Immunity 39, 111–122 (2013).
Gestermann, N. et al. STAT4 is a confirmed genetic risk factor for Sjögren's syndrome and could be involved in type I interferon pathway signaling. Genes Immun. 11, 432–438 (2010).
Dieude, P. et al. STAT4 is a genetic risk factor for systemic sclerosis having additive effects with IRF5 on disease susceptibility and related pulmonary fibrosis. Arthritis Rheum. 60, 2472–2479 (2009).
Remmers, E. F. et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357, 977–986 (2007).
Zervou, M. I., Goulielmos, G. N., Castro-Giner, F., Tosca, A. D. & Krueger-Krasagakis, S. STAT4 gene polymorphism is associated with psoriasis in the genetically homogeneous population of Crete, Greece. Hum. Immunol. 70, 738–741 (2009).
Liang, Y. L. et al. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol. Biol. Rep. 39, 8873–8882 (2012).
Hebert, H. L. et al. Identification of loci associated with late-onset psoriasis using dense genotyping of immune-related regions. Br. J. Dermatol. 172, 933–939 (2015).
Lee, Y. H. et al. The PTPN22 C1858T functional polymorphism and autoimmune diseases — a meta-analysis. Rheumatology (Oxford) 46, 49–56 (2007).
Carlton, V. E. et al. PTPN22 genetic variation: evidence for multiple variants associated with rheumatoid arthritis. Am. J. Hum. Genet. 77, 567–581 (2005).
Mok, A. et al. Genome-wide profiling identifies associations between lupus nephritis and differential methylation of genes regulating tissue hypoxia and type I interferon responses. Lupus Sci. Med. 3, e000183 (2016).
Harley, I. T. W. et al. The role of genetic variation near interferon-κ in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 706825 (2010).
Stannard, J. N. et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J. Invest. Dermatol. 137, 115–122 (2017).
Ko, K., Koldobskaya, Y., Rosenzweig, E. & Niewold, T. B. Activation of the interferon pathway is dependent upon autoantibodies in African-American SLE patients, but not in European-American SLE patients. Frontiers Immunol. 4, 309 (2013).
Hagberg, N. et al. IFN-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes is promoted by NK cells via MIP-1β and LFA-1. J. Immunol. 186, 5085–5094 (2011).
Ghodke-Puranik, Y. et al. Lupus-associated functional polymorphism in PNP causes cell cycle abnormalities and interferon pathway activation in human immune cells. Arthritis Rheumatol. 69, 2328–2337 (2017).
Landolt-Marticorena, C. et al. Lack of association between the interferon-α signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 1440–1446 (2009).
Petri, M. et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus 18, 980–989 (2009).
Bauer, J. W. et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 60, 3098–3107 (2009).
Hjelmervik, T. O., Petersen, K., Jonassen, I., Jonsson, R. & Bolstad, A. I. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren's syndrome patients from healthy control subjects. Arthritis Rheum. 52, 1534–1544 (2005).
Emamian, E. S. et al. Peripheral blood gene expression profiling in Sjögren's syndrome. Genes Immun. 10, 285–296 (2009).
Gottenberg, J. E. et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc. Natl Acad. Sci. USA 103, 2770–2775 (2006).
Niewold, T. B., Rivera, T. L., Buyon, J. P. & Crow, M. K. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum. 58, 541–546 (2008).
Brkic, Z. et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren's syndrome and association with disease activity and BAFF gene expression. Ann. Rheum. Dis. 72, 728–735 (2013).
Li, H. et al. Identification of a Sjögren's syndrome susceptibility locus at OAS1 that influences isoform switching, protein expression, and responsiveness to type I interferons. PLoS Genet. 13, e1006820 (2017).
Vlachogiannis, N. I. et al. Increased frequency of the PTPN22W* variant in primary Sjögren's syndrome: association with low type I IFN scores. Clin. Immunol. 173, 157–160 (2016).
Maria, N. I. et al. Contrasting expression pattern of RNA-sensing receptors TLR7, RIG-I and MDA5 in interferon-positive and interferon-negative patients with primary Sjögren's syndrome. Ann. Rheum. Dis. 76, 721–730 (2017).
Nezos, A. et al. Type I and II interferon signatures in Sjögren's syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjögren's related lymphomagenesis. J. Autoimmun. 63, 47–58 (2015).
Niewold, T. B., Kariuki, S. N., Morgan, G. A., Shrestha, S. & Pachman, L. M. Elevated serum interferon-α activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 60, 1815–1824 (2009).
O'Connor, K. A., Abbott, K. A., Sabin, B., Kuroda, M. & Pachman, L. M. MxA gene expression in juvenile dermatomyositis peripheral blood mononuclear cells: association with muscle involvement. Clin. Immunol. 120, 319–325 (2006).
Shrestha, S. et al. Lesional and non lesional skin from untreated juvenile dermatomyositis (JDM) displays increased mast cells and mature plasmacytoid dendritic cells. Arthritis Rheum. 62, 2813–2822 (2010).
Reed, A. M. et al. Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum. 64, 4078–4086 (2012).
Greenberg, S. A. et al. Relationship between disease activity and type I interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 13, 207–213 (2012).
Niewold, T. B., Kariuki, S. N., Morgan, G. A., Shrestha, S. & Pachman, L. M. Gene-gene-sex interaction in cytokine gene polymorphisms revealed by serum interferon α phenotype in juvenile dermatomyositis. J. Pediatr. 157, 653–657 (2010).
Niewold, T. B., Wu, S. C., Smith, M., Morgan, G. A. & Pachman, L. M. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics 127, e1239–e1246 (2011).
Balboni, I. et al. Detection of anti-Ro, La, Smith and RNP autoantibodies by autoantigen microarray analysis and interferon-α induction in juvenile dermatomyositis. Arthritis Rheum. 2424–2429 (2013).
Dastmalchi, M. et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann. Rheum. Dis. 67, 1670–1677 (2008).
Mavragani, C. P. et al. Augmented interferon-α pathway activation in patients with Sjögren's syndrome treated with etanercept. Arthritis Rheum. 56, 3995–4004 (2007).
Liao, A. P. et al. Interferon β is associated with type I interferon-inducible gene expression in dermatomyositis. Ann. Rheum. Dis. 70, 831–836 (2011).
Tournadre, A., Lenief, V., Eljaafari, A. & Miossec, P. Immature muscle precursors are a source of interferon-β in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis Rheum. 64, 533–541 (2012).
Li, L. et al. Role of Toll-like receptors and retinoic acid inducible gene I in endogenous production of type I interferon in dermatomyositis. J. Neuroimmunol. 285, 161–168 (2015).
Assassi, S. et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 62, 589–598 (2010).
Brkic, Z. et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 75, 1567–1573 (2016).
York, M. R. et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020 (2007).
Christmann, R. B. et al. Association of Interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 66, 714–725 (2014).
Wuttge, D. M. et al. Increased serum type I interferon activity in early systemic sclerosis patients is associated with antibodies against Sjögren's syndrome antigens and nuclear ribonucleoprotein antigens. Scand. J. Rheumatol. 42, 235–240 (2013).
Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput. Biol. 11, e1004005 (2015).
Gorlova, O. et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet. 7, e1002178 (2011).
Barizzone, N. et al. Rare variants in the TREX1 gene and susceptibility to autoimmune diseases. Biomed. Res. Int. 2013, 471703 (2013).
van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014).
Aidoudi, S., Bujakowska, K., Kieffer, N. & Bikfalvi, A. The CXC-chemokine CXCL4 interacts with integrins implicated in angiogenesis. PLoS ONE 3, e2657 (2008).
Romagnani, P. et al. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on T(H)1 or T-H cytokine production. J. Allergy Clin. Immunol. 116, 1372–1379 (2005).
Liu, C. Y. et al. Platelet factor 4 differentially modulates CD4(+)CD25(+) (regulatory) versus CD4(+)CD25(-) (nonregulatory) T cells. J. Immunol. 174, 2680–2686 (2005).
Stevens, W., Vancheeswaran, R. & Black, C. M. Alpha interferon-2a (Roferon-A) in the treatment of diffuse cutaneous systemic sclerosis: a pilot study. Br. J. Rheumatol 31, 683–689 (1992).
Black, C. M. et al. Interferon-α does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 42, 299–305 (1999).
Lubbers, J. et al. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann. Rheum. Dis. 72, 776–780 (2013).
Hua, J., Kirou, K., Lee, C. & Crow, M. K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 54, 1906–1916 (2006).
Han, T. U. et al. Association of an activity-enhancing variant of IRAK1 and an MECP2-IRAK1 haplotype with increased susceptibility to rheumatoid arthritis. Arthritis Rheum. 65, 590–598 (2013).
Orozco, G. et al. Association of a functional single-nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 52, 219–224 (2005).
Lande, R. et al. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 173, 2815–2824 (2004).
Cavanagh, L. L. et al. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res. Ther. 7, R230–240 (2005).
van Holten, J., Smeets, T. J., Blankert, P. & Tak, P. P. Expression of interferon β in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann. Rheum. Dis. 64, 1780–1782 (2005).
Roelofs, M. F. et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Ann. Rheum. Dis. 68, 1486–1493 (2009).
Coclet-Ninin, J., Dayer, J. M. & Burger, D. Interferon-β not only inhibits interleukin-1β and tumor necrosis factor-α but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur. Cytokine Netw 8, 345–349 (1997).
Palmer, G. et al. Interferon β stimulates interleukin 1 receptor antagonist production in human articular chondrocytes and synovial fibroblasts. Ann. Rheum. Dis. 63, 43–49 (2004).
Triantaphyllopoulos, K. A., Williams, R. O., Tailor, H. & Chernajovsky, Y. Amelioration of collagen-induced arthritis and suppression of interferon-γ, interleukin-12, and tumor necrosis factor alpha production by interferon-β gene therapy. Arthritis Rheum. 42, 90–99 (1999).
van Holten, J. et al. Treatment with recombinant interferon-β reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 6, R239–R249 (2004).
van Holten, J. et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon β-1a in the treatment of patients with active rheumatoid arthritis. Ann. Rheum. Dis. 64, 64–69 (2005).
Raterman, H. G. et al. The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res. Ther. 14, R95 (2012).
Mavragani, C. P., La, D. T., Stohl, W. & Crow, M. K. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-β/α ratios in rheumatoid arthritis patients: a post hoc analysis of a predominantly Hispanic cohort. Arthritis Rheum. 62, 392–401 (2010).
Wampler Muskardin, T. et al. Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis. Ann. Rheum. Dis. 75, 1757–1762 (2016).
de Jong, T. D. et al. Physiological evidence for diversification of IFNα- and IFNβ-mediated response programs in different autoimmune diseases. Arthritis Res. Ther. 18, 49 (2016).
Coclet-Ninin, J., Dayer, J. M. & Burger, D. Interferon-β not only inhibits interleukin-1 β and tumor necrosis factor-α but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur. Cytokine Netw. 8, 345–349 (1997).
Kim, H., Sanchez, G. A. & Goldbach-Mansky, R. Insights from Mendelian interferonopathies: comparison of CANDLE, SAVI with AGS, monogenic lupus. J. Mol. Med. 94, 1111–1127 (2016).
Paludan, S. R. & Bowie, A. G. Immune sensing of DNA. Immunity 38, 870–880 (2013).
Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 12, 270–279 (2011).
Rice, G. et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am. J. Hum. Genet. 80, 811–815 (2007).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Rice, G. I., Rodero, M. P. & Crow, Y. J. Human disease phenotypes associated with mutations in TREX1. J. Clin. Immunol. 35, 235–243 (2015).
Jang, M. A. et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am. J. Hum. Genet. 96, 266–274 (2015).
Torrelo, A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol. 8, 927 (2017).
Brehm, A. et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Invest. 125, 4196–4211 (2015).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Warner, J. D. et al. STING-associated vasculopathy develops independently of IRF3 in mice. J. Exp. Med. https://doi.org/10.1084/jem.20171351 (2017).
Merrill, J. T. et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 70, 1905–1913 (2011).
McBride, J. M. et al. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 64, 3666–3676 (2012).
Petri, M. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65, 1011–1021 (2013).
Niewold, T. B. Connective tissue diseases: Targeting type I interferon in systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 377–378 (2016).
Khamashta, M. et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Lauwerys, B. R. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 65, 447–456 (2013).
Higgs, B. W. et al. A phase Ib clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann. Rheum. Dis. 73, 256–262 (2014).
Peng, L., Oganesyan, V., Wu, H., Dall'Acqua, W. F. & Damschroder, M. M. Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-α receptor 1 antibody. mAbs 7, 428–439 (2015).
Furie, R. et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
Guo, X. et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J. Invest. Dermatol. 135, 2402–2409 (2015).
Bialas, A. R. et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 546, 539–543 (2017).
Sacre, K., Criswell, L. A. & McCune, J. M. Hydroxychloroquine is associated with impaired interferon-α and tumor necrosis factor-α production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 14, R155 (2012).
Alarcon, G. S. et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 66, 1168–1172 (2007).
Bruce, I. N. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann. Rheum. Dis. 74, 1706–1713 (2015).
Rempenault, C. et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2017-211836 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03085940 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02595346 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01379573 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03030118 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02603146 (2017).
Postal, M., Sinicato, N. A., Appenzeller, S. & Niewold, T. B. Drugs in early clinical development for systemic lupus erythematosus. Expert Opin. Invest. Drugs 25, 573–583 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03241108 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02592434 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01500551 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03000439 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01976364 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01882439 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01877668 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03159936 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02535689 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03288324 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03002649 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02265705 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01721044 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01885078 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01721057 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01710358 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01711359 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01724580 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02065700 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01894516 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01888874 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01668641 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01384422 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02885181 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03320876 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03101670 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03285711 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02996500 (2018).
Liang, Y. et al. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat. Immunol. 18, 152–160 (2017).
Canosi, U. et al. A highly precise reporter gene bioassay for type I interferon. J. Immunol. Methods 199, 69–76 (1996).
Khabar, K. S. et al. Expressed gene clusters associated with cellular sensitivity and resistance towards anti-viral and anti-proliferative actions of interferon. J. Mol. Biol. 342, 833–846 (2004).
Niewold, T. B., Clark, D. N., Salloum, R. & Poole, B. D. Interferon α in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 948364 (2010).
Acknowledgements
The work of T.L.W.M. is supported by grants from the Central Society for Clinical and Translational Research. The work of T.B.N. is supported by grants from the NIH (AR060861, AR057781and AR065964), the Rheumatology Research Foundation, CureJM Foundation, the Myositis Association, the Lupus Research Alliance and the Colton Center for Autoimmunity.
Author information
Authors and Affiliations
Contributions
Both authors wrote the article, provided substantial contributions to discussions of its content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.B.N. declares that he has received research grants from EMD Serono and Janssen. T.L.W.N declares no competing interests.
Rights and permissions
About this article
Cite this article
Muskardin, T., Niewold, T. Type I interferon in rheumatic diseases. Nat Rev Rheumatol 14, 214–228 (2018). https://doi.org/10.1038/nrrheum.2018.31
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.31
This article is cited by
-
Efficacy of Ban-Qin-Fei-Re-Qing oral liquid on avian infectious bronchitis
One Health Advances (2024)
-
Role of IFN-α in Rheumatoid Arthritis
Current Rheumatology Reports (2024)
-
Relationship of systemic type I interferon activity with clinical phenotypes, disease activity, and damage accrual in systemic lupus erythematosus in treatment-naive patients: a retrospective longitudinal analysis
Arthritis Research & Therapy (2023)
-
A multi-center, open-label, randomized study to explore efficacy and safety of baricitinib in active primary Sjogren’s syndrome patients
Trials (2023)
-
Lactobacillus acidophilus and propionate attenuate Sjögren’s syndrome by modulating the STIM1-STING signaling pathway
Cell Communication and Signaling (2023)