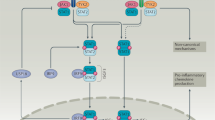
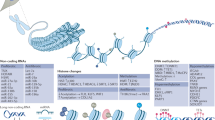
Abstract
Type I interferons have been suspected for decades to have a crucial role in the pathogenesis of systemic lupus erythematosus (SLE). Evidence has now overturned several long-held assumptions about how type I interferons are regulated and cause pathological conditions, providing a new view of SLE pathogenesis that resolves longstanding clinical dilemmas. This evidence includes data on interferons in relation to genetic predisposition and epigenetic regulation. Importantly, data are now available on the role of interferons in the early phases of the disease and the importance of non-haematopoietic cellular sources of type I interferons, such as keratinocytes, renal tubular cells, glial cells and synovial stromal cells, as well as local responses to type I interferons within these tissues. These local effects are found not only in inflamed target organs in established SLE, but also in histologically normal skin during asymptomatic preclinical phases, suggesting a role in disease initiation. In terms of clinical application, evidence relating to biomarkers to characterize the type I interferon system is complex, and, notably, interferon-blocking therapies are now licensed for the treatment of SLE. Collectively, the available data enable us to propose a model of disease pathogenesis that invokes the unique value of interferon-targeted therapies. Accordingly, future approaches in SLE involving disease reclassification and preventative strategies in preclinical phases should be investigated.
Key points
-
Type I interferon pathway variants are prominent in genetic predisposition to systemic lupus erythematosus (SLE), and epigenetic regulation affects classic interferon-stimulated genes and genes less directly related to interferon signalling.
-
Contrary to previous assumptions, plasmacytoid dendritic cells lose their immunogenic functions in SLE, including type I interferon production and antigen presentation.
-
Non-haematopoietic cells within organs affected by SLE (especially the skin) produce type I interferons, express interferon-stimulated genes, and have roles in the initiation of SLE and in established SLE.
-
Both the dysfunction of plasmacytoid dendritic cells and the non-haematopoietic production of type I interferons is seen in all anti-nuclear antibody-positive individuals, regardless of whether progression to organ inflammation later occurs.
-
Diverse biomarkers (each with advantages and disadvantages) can characterize type I interferon pathway activation, including evaluation of interferon proteins and several effects downstream of the type I interferon receptor.
-
Several therapies targeting the type I interferon pathway are in development (including the now licensed anifrolumab), and might have specific clinical properties relating to the model of SLE proposed herein.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Psarras, A., Emery, P. & Vital, E. M. Type I interferon-mediated autoimmune diseases: pathogenesis, diagnosis and targeted therapy. Rheumatology 56, 1662–1675 (2017).
Ronnblom, L. & Alm, G. V. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 22, 427–431 (2001).
Walling, H. W. & Sontheimer, R. D. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am. J. Clin. Dermatol. 10, 365–381 (2009).
Vital, E. M. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 63, 3038–3047 (2011).
Vital, E. M. et al. Brief report: responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol. 67, 1586–1591 (2015).
Furie, R. A. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 1, e208–e219 (2019).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Eriksson, C. et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res. Ther. 13, R30 (2011).
Wandstrat, A. E. et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J. Autoimmun. 27, 153–160 (2006).
Niewold, T. B., Hua, J., Lehman, T. J., Harley, J. B. & Crow, M. K. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 8, 492–502 (2007).
Niewold, T. B. et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann. Rheum. Dis. 71, 463–468 (2012).
Niewold, T. B. et al. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 58, 2481–2487 (2008).
El-Sherbiny, Y. M. et al. A novel two-score system for interferon status segregates autoimmune diseases and correlates with clinical features. Sci. Rep. 8, 5793 (2018).
Lu, R. et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J. Autoimmun. 74, 182–193 (2016).
Langkilde, H., Voss, A., Heegaard, N. & Laustrup, H. Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 26, 723–728 (2017).
Munroe, M. E. et al. Pathways of impending disease flare in African-American systemic lupus erythematosus patients. J. Autoimmun. 78, 70–78 (2017).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Md Yusof, M. Y. et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann. Rheum. Dis. 77, 1432–1439 (2018).
Deng, Y. & Tsao, B. P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6, 683–692 (2010).
Wang, Y. F. et al. Identification of 38 novel loci for systemic lupus erythematosus and genetic heterogeneity between ancestral groups. Nat. Commun. 12, 772 (2021).
Graham, R. R. et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38, 550–555 (2006).
Feng, D. et al. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum. 62, 562–573 (2010).
International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN). et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Salloum, R. et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthritis Rheum. 62, 553–561 (2010).
Robinson, T. et al. Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-alpha and serologic autoimmunity in lupus patients. J. Immunol. 187, 1298–1303 (2011).
Remmers, E. F. et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357, 977–986 (2007).
Kariuki, S. N. et al. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J. Immunol. 182, 34–38 (2009).
Harley, I. T. et al. The role of genetic variation near interferon-kappa in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 706825 (2010).
Panousis, N. I. et al. Combined genetic and transcriptome analysis of patients with SLE: distinct, targetable signatures for susceptibility and severity. Ann. Rheum. Dis. 78, 1079–1089 (2019).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Kamada, R. et al. Interferon stimulation creates chromatin marks and establishes transcriptional memory. Proc. Natl Acad. Sci. USA 115, E9162–E9171 (2018).
Park, S. H. et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 18, 1104–1116 (2017).
Care, M. A. et al. Network analysis identifies proinflammatory plasma cell polarization for secretion of ISG15 in human autoimmunity. J. Immunol. 197, 1447–1459 (2016).
Eloranta, M. L., Alm, G. V. & Ronnblom, L. Disease mechanisms in rheumatology-tools and pathways: plasmacytoid dendritic cells and their role in autoimmune rheumatic diseases. Arthritis Rheum. 65, 853–863 (2013).
Vallin, H., Perers, A., Alm, G. V. & Ronnblom, L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-α inducer in systemic lupus erythematosus. J. Immunol. 163, 6306–6313 (1999).
Vallin, H., Blomberg, S., Alm, G. V., Cederblad, B. & Ronnblom, L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-α) production acting on leucocytes resembling immature dendritic cells. Clin. Exp. Immunol. 115, 196–202 (1999).
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Blomberg, S., Eloranta, M. L., Magnusson, M., Alm, G. V. & Ronnblom, L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-α by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 48, 2524–2532 (2003).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Cao, W. & Bover, L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol. Rev. 234, 163–176 (2010).
Psarras, A. et al. TNF-α regulates human plasmacytoid dendritic cells by suppressing IFN-α production and enhancing T cell activation. J. Immunol. 206, 785–796 (2021).
Macal, M. et al. Self-renewal and Toll-like receptor signaling sustain exhausted plasmacytoid dendritic cells during chronic viral infection. Immunity 48, 730–744.e5 (2018).
Moisini, I. et al. The Yaa locus and IFN-α fine-tune germinal center B cell selection in murine systemic lupus erythematosus. J. Immunol. 189, 4305–4312 (2012).
Dai, C. et al. Interferon alpha on NZM2328.Lc1R27: enhancing autoimmunity and immune complex-mediated glomerulonephritis without end stage renal failure. Clin. Immunol. 154, 66–71 (2014).
Sriram, U. et al. Myeloid dendritic cells from B6.NZM Sle1/Sle2/Sle3 lupus-prone mice express an IFN signature that precedes disease onset. J. Immunol. 189, 80–91 (2012).
Hadj-Slimane, R., Chelbi-Alix, M. K., Tovey, M. G. & Bobe, P. An essential role for IFN-α in the overexpression of Fas ligand on MRL/lpr lymphocytes and on their spontaneous Fas-mediated cytotoxic potential. J. Interferon Cytokine Res. 24, 717–728 (2004).
Zhou, Z. et al. Phenotypic and functional alterations of pDCs in lupus-prone mice. Sci. Rep. 6, 20373 (2016).
Liao, X. et al. Cutting edge: plasmacytoid dendritic cells in late-stage lupus mice defective in producing IFN-α. J. Immunol. 195, 4578–4582 (2015).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Rodero, M. P. et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J. Exp. Med. 214, 1547–1555 (2017).
Psarras, A. et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 11, 6149 (2020).
Iwamoto, T. et al. High systemic type I interferon activity is associated with active class III/IV lupus nephritis. J. Rheumatol. 49, 388–397 (2022).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Skopelja-Gardner, S. et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl Acad. Sci. USA 118, e2019097118 (2021).
Skopelja-Gardner, S. et al. The early local and systemic type I interferon responses to ultraviolet B light exposure are cGAS dependent. Sci. Rep. 10, 7908 (2020).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184, 4464–4479.e19 (2021).
LaFleur, D. W. et al. Interferon-κ, a novel type I interferon expressed in human keratinocytes. J. Biol. Chem. 276, 39765–39771 (2001).
Sarkar, M. K. et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann. Rheum. Dis. 77, 1653–1664 (2018).
Stannard, J. N. et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J. Invest. Dermatol. 137, 115–122 (2017).
Tsoi, L. C. et al. Hypersensitive IFN responses in lupus keratinocytes reveal key mechanistic determinants in cutaneous lupus. J. Immunol. 202, 2121–2130 (2019).
Shalbaf, M. et al. Plucked hair follicles from patients with chronic discoid lupus erythematosus show a disease-specific molecular signature. Lupus Sci. Med. 6, e000328 (2019).
Naik, S. et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480 (2017).
Alase, A. A. et al. IFNλ stimulates MxA production in human dermal fibroblasts via a MAPK-dependent STAT1-independent mechanism. J. Invest. Dermatol. 135, 2935–2943 (2015).
Billi, A. C. et al. Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci. Transl. Med. 14, eabn2263 (2022).
Castellano, G. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 17, 72 (2015).
Benigni, A. et al. Involvement of renal tubular Toll-like receptor 9 in the development of tubulointerstitial injury in systemic lupus. Arthritis Rheum. 56, 1569–1578 (2007).
Papadimitraki, E. D., Tzardi, M., Bertsias, G., Sotsiou, E. & Boumpas, D. T. Glomerular expression of toll-like receptor-9 in lupus nephritis but not in normal kidneys: implications for the amplification of the inflammatory response. Lupus 18, 831–835 (2009).
Der, E. et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight 2, e93009 (2017).
Chiche, L. et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 66, 1583–1595 (2014).
Nzeusseu Toukap, A. et al. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 56, 1579–1588 (2007).
van Holten, J., Smeets, T. J., Blankert, P. & Tak, P. P. Expression of interferon β in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann. Rheum. Dis. 64, 1780–1782 (2005).
van Holten, J. et al. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 6, R239–R249 (2004).
Adriaansen, J. et al. Intraarticular interferon-β gene therapy ameliorates adjuvant arthritis in rats. Hum. Gene Ther. 17, 985–996 (2006).
Treschow, A. P., Teige, I., Nandakumar, K. S., Holmdahl, R. & Issazadeh-Navikas, S. Stromal cells and osteoclasts are responsible for exacerbated collagen-induced arthritis in interferon-β-deficient mice. Arthritis Rheum. 52, 3739–3748 (2005).
Mensah, K. A. et al. Mediation of nonerosive arthritis in a mouse model of lupus by interferon-α-stimulated monocyte differentiation that is nonpermissive of osteoclastogenesis. Arthritis Rheum. 62, 1127–1137 (2010).
Mahmoud, K., Zayat, A. & Vital, E. M. Musculoskeletal manifestations of systemic lupus erythmatosus. Curr. Opin. Rheumatol. 29, 486–492 (2017).
Merrill, J. T. et al. Anifrolumab effects on rash and arthritis: impact of the type I interferon gene signature in the phase IIb MUSE study in patients with systemic lupus erythematosus. Lupus Sci. Med. 5, e000284 (2018).
McGlasson, S., Jury, A., Jackson, A. & Hunt, D. Type I interferon dysregulation and neurological disease. Nat. Rev. Neurol. 11, 515–523 (2015).
Crow, Y. J. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi–Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Rice, G. I. et al. Mutations involved in Aicardi–Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009).
Mannion, N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014).
Rice, G. I. et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 (2014).
Dale, R. C., Tang, S. P., Heckmatt, J. Z. & Tatnall, F. M. Familial systemic lupus erythematosus and congenital infection-like syndrome. Neuropediatrics 31, 155–158 (2000).
De Laet, C. et al. Phenotypic overlap between infantile systemic lupus erythematosus and Aicardi–Goutieres syndrome. Neuropediatrics 36, 399–402 (2005).
Lee-Kirsch, M. A. et al. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J. Mol. Med. 85, 531–537 (2007).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Lafaille, F. G. et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491, 769–773 (2012).
Crow, Y. J. et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi–Goutieres syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 (2006).
Cuadrado, E. et al. Phenotypic variation in Aicardi–Goutieres syndrome explained by cell-specific IFN-stimulated gene response and cytokine release. J. Immunol. 194, 3623–3633 (2015).
van Heteren, J. T. et al. Astrocytes produce interferon-α and CXCL10, but not IL-6 or CXCL8, in Aicardi–Goutieres syndrome. Glia 56, 568–578 (2008).
Hunt, D. et al. Thrombotic microangiopathy associated with interferon beta. N. Engl. J. Med. 370, 1270–1271 (2014).
Kavanagh, D. et al. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood 128, 2824–2833 (2016).
Heinze, S. et al. Depressive mood changes and psychiatric symptoms during 12-month low-dose interferon-α treatment in patients with malignant melanoma: results from the multicenter DeCOG trial. J. Immunother. 33, 106–114 (2010).
Lebon, P., Lenoir, G. R., Fischer, A. & Lagrue, A. Synthesis of intrathecal interferon in systemic lupus erythematosus with neurological complications. Br. Med. J. (Clin. Res. Ed.) 287, 1165–1167 (1983).
Shiozawa, S., Kuroki, Y., Kim, M., Hirohata, S. & Ogino, T. Interferon-α in lupus psychosis. Arthritis Rheum. 35, 417–422 (1992).
McGlasson, S., Wiseman, S., Wardlaw, J., Dhaun, N. & Hunt, D. P. J. Neurological disease in lupus: toward a personalized medicine approach. Front. Immunol. 9, 1146 (2018).
Tyden, H. et al. Endothelial dysfunction is associated with activation of the type I interferon system and platelets in patients with systemic lupus erythematosus. RMD Open 3, e000508 (2017).
Lee, P. Y. et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 56, 3759–3769 (2007).
Ding, X., Xiang, W. & He, X. IFN-I mediates dysfunction of endothelial progenitor cells in atherosclerosis of systemic lupus erythematosus. Front. Immunol. 11, 581385 (2020).
Wiseman, S. J. et al. Cerebral small vessel disease burden is increased in systemic lupus erythematosus. Stroke 47, 2722–2728 (2016).
Sibbitt, W. L. Jr. et al. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin. Arthritis Rheum. 40, 32–52 (2010).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Hua, J., Kirou, K., Lee, C. & Crow, M. K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 54, 1906–1916 (2006).
Mathian, A. et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital enzyme-linked immunosorbent assay quantification of serum interferon-α. Arthritis Rheumatol. 71, 756–765 (2019).
Mathian, A. et al. Ultrasensitive serum interferon-α quantification during SLE remission identifies patients at risk for relapse. Ann. Rheum. Dis. 78, 1669–1676 (2019).
Higgs, B. W. et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 70, 2029–2036 (2011).
Kennedy, W. P. et al. Association of the interferon signature metric with serological disease manifestations but not global activity scores in multiple cohorts of patients with SLE. Lupus Sci. Med. 2, e000080 (2015).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Chatterjee-Kishore, M., Wright, K. L., Ting, J. P. & Stark, G. R. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. 19, 4111–4122 (2000).
Qiao, Y. et al. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity 39, 454–469 (2013).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Alase, A. et al. Prediction of response to rituximab in SLE using a validated two-score system for interferon. Ann. Rheum. Dis. 78, 763–764 (2019).
Bauer, J. W. et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 3, e491 (2006).
Metzemaekers, M., Vanheule, V., Janssens, R., Struyf, S. & Proost, P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front. Immunol. 8, 1970 (2017).
Saber, M. A., Okasha, H., Khorshed, F. & Samir, S. A novel cell-based in vitro assay for antiviral activity of interferons alpha, beta, and gamma by qPCR of MxA gene expression. Recent. Pat. Biotechnol. 15, 67–75 (2021).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
El-Sherbiny, Y. M. et al. B cell tetherin: a flow cytometric cell-specific assay for response to type I interferon predicts clinical features and flares in systemic lupus erythematosus. Arthritis Rheumatol. 72, 769–779 (2020).
Biesen, R. et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 58, 1136–1145 (2008).
Becker, A. M. et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One 8, e67003 (2013).
Whitney, A. R. et al. Individuality and variation in gene expression patterns in human blood. Proc. Natl Acad. Sci. USA 100, 1896–1901 (2003).
Fayyaz, A. et al. Haematological manifestations of lupus. Lupus Sci. Med. 2, e000078 (2015).
Jacobi, A. M. et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 305–308 (2010).
Md Yusof, M. Y. et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann. Rheum. Dis. 76, 1829–1836 (2017).
Zhu, H. et al. Whole-genome transcription and DNA methylation analysis of peripheral blood mononuclear cells identified aberrant gene regulation pathways in systemic lupus erythematosus. Arthritis Res. Ther. 18, 162 (2016).
Preble, O. T., Black, R. J., Friedman, R. M., Klippel, J. H. & Vilcek, J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 216, 429–431 (1982).
Arvin, A. M. & Miller, J. J. 3rd Acid labile α-interferon in sera and synovial fluids from patients with juvenile arthritis. Arthritis Rheum. 27, 582–585 (1984).
Furie, R. et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017).
Petri, M. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65, 1011–1021 (2013).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Ducreux, J. et al. Interferon α kinoid induces neutralizing anti-interferon α antibodies that decrease the expression of interferon-induced and B cell activation associated transcripts: analysis of extended follow-up data from the interferon α kinoid phase I/II study. Rheumatology 55, 1901–1905 (2016).
Lauwerys, B. R. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 65, 447–456 (2013).
Morehouse, C. et al. Target modulation of a type I interferon (IFN) gene signature with sifalimumab or anifrolumab in systemic lupus erythematosus (SLE) patients in two open label phase 2 Japanese trials. Arthritis Rheumatol. 66, S1–S1402 (2014).
Furie, R. et al. Anifrolumab reduces flare rates in patients with moderate to severe systemic lupus erythematosus. Lupus 30, 1254–1263 (2021).
Abdel Galil, S. M., El-Shafey, A. M., Abdul-Maksoud, R. S. & El-Boshy, M. Interferon α gene expression and serum level association with low vitamin D levels in Egyptian female patients with systemic lupus erythematosus. Lupus 27, 199–209 (2018).
Zayat, A. S. et al. Defining inflammatory musculoskeletal manifestations in systemic lupus erythematosus. Rheumatology 58, 304–312 (2019).
Mahmoud, K. et al. Responsiveness of clinical and ultrasound outcome measures in musculoskeletal systemic lupus erythematosus. Rheumatology 58, 1353–1360 (2019).
Mahmoud, K. et al. Ultrasound to identify systemic lupus erythematosus patients with musculoskeletal symptoms who respond best to therapy: the US Evaluation For mUsculoskeletal Lupus longitudinal multicentre study. Rheumatology 60, 5194–5204 (2021).
Vanderver, A. et al. Janus kinase inhibition in the Aicardi-Goutieres syndrome. N. Engl. J. Med. 383, 986–989 (2020).
Wenzel, J. et al. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J. Invest. Dermatol. 136, 1281–1283 (2016).
Hasni, S. A. et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 12, 3391 (2021).
Wallace, D. J. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392, 222–231 (2018).
Dorner, T. et al. Baricitinib-associated changes in global gene expression during a 24-week phase II clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci. Med. 7, e000424 (2020).
US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03845517ClinicalTrials.gov (2022).
Oon, S. et al. A cytotoxic anti-IL-3Rα antibody targets key cells and cytokines implicated in systemic lupus erythematosus. JCI Insight 1, e86131 (2016).
Hartmann, S. et al. A clinical population pharmacokinetic/pharmacodynamic model for BIIB059, a monoclonal antibody for the treatment of systemic and cutaneous lupus erythematosus. J. Pharmacokinet. Pharmacodyn. 47, 255–266 (2020).
Werth, V. et al. OP0193 BIIB059, A humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (PDC), shows dose-related efficacy in the phase 2 LILAC study in patients (PTS) with active cutaneous lupus erythematosus (CLE). Ann. Rheum. Dis. 79, 120 (2020).
Furie, R. et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Invest. 129, 1359–1371 (2019).
Hamilton, J. A., Hsu, H. C. & Mountz, J. D. Role of production of type I interferons by B cells in the mechanisms and pathogenesis of systemic lupus erythematosus. Discov. Med. 25, 21–29 (2018).
Patel, J., Maddukuri, S., Li, Y., Bax, C. & Werth, V. P. Highly multiplexed mass cytometry identifies the immunophenotype in the skin of dermatomyositis. J. Invest. Dermatol. 141, 2151–2160 (2021).
Testa, U., Pelosi, E. & Frankel, A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark. Res. 2, 4 (2014).
Li, Y. et al. Monocyte surface expression of Fcγ receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res. Ther. 12, R90 (2010).
Kawasaki, M. et al. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus 20, 1231–1239 (2011).
Lambers, W. M., Westra, J., Bootsma, H. & de Leeuw, K. Hydroxychloroquine suppresses interferon-inducible genes and B cell activating factor in patients with incomplete and new-onset systemic lupus erythematosus. J. Rheumatol. 48, 847–851 (2021).
Khamashta, M. et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Houssiau, F. A. et al. IFN-α kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 79, 347–355 (2020).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
E.M.V. has received research grants from AstraZeneca and Sandoz and honoraria from AstraZeneca, Aurinia, GSK, Modus, Lilly and Roche. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Psarras, A., Wittmann, M. & Vital, E.M. Emerging concepts of type I interferons in SLE pathogenesis and therapy. Nat Rev Rheumatol 18, 575–590 (2022). https://doi.org/10.1038/s41584-022-00826-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00826-z