Abstract
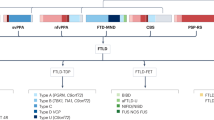
Frontotemporal lobar degeneration (FTLD) encompasses a group of diseases characterized by neuronal loss and gliosis of the frontal and temporal lobes. Almost all cases of FTLD can be classified into three categories on the basis of deposition of one of three abnormal proteins: the microtubule-associated protein tau, TAR DNA-binding protein 43, or fused in sarcoma. The specific diagnoses within each of these three categories are further differentiated by the distribution and morphological appearance of the protein-containing inclusions. Future treatments are likely to target these abnormal proteins; the clinical challenge, therefore, is to be able to predict molecular pathology during life. Clinical diagnosis alone has had variable success in helping to predict pathology, and is particularly poor in the diagnosis of behavioral variant frontotemporal dementia, which can be associated with all three abnormal proteins. Consequently, other biomarkers of disease are needed. This Review highlights how patterns of atrophy assessed on MRI demonstrate neuroanatomical signatures of the individual FTLD pathologies, independent of clinical phenotype. The roles of these patterns of atrophy as biomarkers of disease, and their potential to help predict pathology during life in patients with FTLD, are also discussed.
Key Points
-
The term frontotemporal lobar degeneration (FTLD) encompasses a group of pathological disorders that can be characterized by deposition of abnormal forms of tau, TAR DNA-binding protein 43 or fused in sarcoma
-
Predicting the underlying pathology in patients with FTLD will be particularly important when treatments become available that target these abnormal proteins
-
Neuroimaging studies in patients with autopsy-confirmed FTLD have shown that the different pathological diagnoses have specific neuroanatomical signatures
-
The imaging signatures of pathology in patients with FTLD disorders may be useful for predicting the underlying pathology in these individuals
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Josephs, K. A. Frontotemporal dementia and related disorders: deciphering the enigma. Ann. Neurol. 64, 4–14 (2008).
Josephs, K. A. et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 122, 137–153 (2011).
Mackenzie, I. R. et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 119, 1–4 (2010).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Neumann, M. et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132, 2922–2931 (2009).
Hutton, M. et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Dejesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 (2004).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008).
Kwiatkowski, T. J. Jr et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
Skibinski, G. et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet 37, 806–808 (2005).
Forman, M. S. et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J. Neuropathol. Exp. Neurol. 65, 571–581 (2006).
Neumann, M. et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J. Neuropathol. Exp. Neurol. 66, 152–157 (2007).
Holm, I. E., Isaacs, A. M. & Mackenzie, I. R. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol. 118, 719–720 (2009).
Snowden, J. S. et al. The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene. Acta Neuropathol. 122, 99–110 (2011).
Josephs, K. A. et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66, 41–48 (2006).
Hodges, J. R. et al. Clinicopathological correlates in frontotemporal dementia. Ann. Neurol. 56, 399–406 (2004).
Josephs, K. A. et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129, 1385–1398 (2006).
Deramecourt, V. et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 74, 42–49 (2010).
Forman, M. S. et al. Frontotemporal dementia: clinicopathological correlations. Ann. Neurol. 59, 952–962 (2006).
Kertesz, A., McMonagle, P., Blair, M., Davidson, W. & Munoz, D. G. The evolution and pathology of frontotemporal dementia. Brain 128, 1996–2005 (2005).
Vemuri, P. et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage 42, 559–567 (2008).
Whitwell, J. L. et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71, 743–749 (2008).
Dickson, D. W. et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J. Neuropathol. Exp. Neurol. 61, 935–946 (2002).
Kouri, N., Whitwell, J. L., Josephs, K. A., Rademakers, R. & Dickson, D. W. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat. Rev. Neurol. 7, 263–272 (2011).
Hauw, J. J. et al. Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44, 2015–2019 (1994).
Dickson, D. W. Neuropathology of Pick's disease. Neurology 56, S16–S20 (2001).
Bigio, E. H. et al. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J. Neuropathol. Exp. Neurol. 60, 328–341 (2001).
Kovacs, G. G. et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J. Neuropathol. Exp. Neurol. 67, 963–975 (2008).
Braak, H. & Braak, E. Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci. Lett. 76, 124–127 (1987).
Ishihara, K. et al. Argyrophilic grain disease presenting with frontotemporal dementia: a neuropsychological and pathological study of an autopsied case with presenile onset. Neuropathology 25, 165–170 (2005).
Josephs, K. A. et al. Argyrophilic grains: a distinct disease or an additive pathology? Neurobiol. Aging 29, 566–573 (2008).
Boeve, B. F. et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 53, 795–800 (1999).
Josephs, K. A. et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol. Aging 29, 280–289 (2008).
Whitwell, J. L. et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 75, 1879–1887 (2010).
Whitwell, J. L. et al. Imaging signatures of molecular pathology in behavioral variant frontotemporal dementia. J. Mol. Neurosci. 45, 372–378 (2011).
Josephs, K. A. et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch. Neurol. 61, 1881–1884 (2004).
Rankin, K. P. et al. Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick's disease. J. Mol. Neurosci. 45, 594–608 (2011).
Rohrer, J. D. et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134, 2565–2581 (2011).
Whitwell, J. L. et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain 130, 1148–1158 (2007).
Slowinski, J. et al. MR imaging of brainstem atrophy in progressive supranuclear palsy. J. Neurol. 255, 37–44 (2008).
Tsuboi, Y. et al. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 60, 1766–1769 (2003).
Lee, S. E. et al. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 70, 327–340 (2011).
Tokumaru, A. M. et al. Imaging–pathologic correlation in corticobasal degeneration. AJNR Am. J. Neuroradiol. 30, 1884–1892 (2009).
Hassan, A. et al. Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat. Disord. 16, 208–214 (2010).
Schrag, A. et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 54, 697–702 (2000).
Lieberman, A. P. et al. Cognitive, neuroimaging, and pathological studies in a patient with Pick's disease. Ann. Neurol. 43, 259–265 (1998).
Whitwell, J. L. et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch. Neurol. 62, 1402–1408 (2005).
Piguet, O. et al. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology 76, 253–259 (2011).
Arima, K. et al. Two brothers with frontotemporal dementia and parkinsonism with an N279K mutation of the tau gene. Neurology 54, 1787–1795 (2000).
Bird, T. D. et al. A clinical pathological comparison of three families with frontotemporal dementia and identical mutations in the tau gene (P301L). Brain 122, 741–756 (1999).
Boeve, B. F. et al. Longitudinal characterization of two siblings with frontotemporal dementia and parkinsonism linked to chromosome 17 associated with the S305N tau mutation. Brain 128, 752–772 (2005).
Frank, A. R., Wszolek, Z. K., Jack, C. R. Jr & Boeve, B. F. Distinctive MRI findings in pallidopontonigral degeneration (PPND). Neurology 68, 620–621 (2007).
Ghetti, B. et al. In vivo and postmortem clinicoanatomical correlations in frontotemporal dementia and parkinsonism linked to chromosome 17. Neurodegener. Dis. 5, 215–217 (2008).
Janssen, J. C. et al. Clinical features of frontotemporal dementia due to the intronic tau 10+16 mutation. Neurology 58, 1161–1168 (2002).
Tolnay, M. et al. A new case of frontotemporal dementia and parkinsonism resulting from an intron 10 +3-splice site mutation in the tau gene: clinical and pathological features. Neuropathol. Appl. Neurobiol. 26, 368–378 (2000).
van Swieten, J. C. et al. Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann. Neurol. 46, 617–626 (1999).
Rohrer, J. D. et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage 53, 1070–1076 (2010).
Spina, S. et al. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain 131, 72–89 (2008).
Whitwell, J. L. et al. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology 73, 1058–1065 (2009).
Whitwell, J. L. et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 72, 813–820 (2009).
Whitwell, J. L. & Josephs, K. A. Imaging in familial frontotemporal lobar degeneration with mutations in MAPT or PGRN. Eur. Neurol. J. 1, 25–31 (2009).
Slowinski, J. L. et al. Brainstem atrophy on routine MR study in pallidopontonigral degeneration. J. Neurol. 256, 827–829 (2009).
Soliveri, P. et al. A case of dementia parkinsonism resembling progressive supranuclear palsy due to mutation in the tau protein gene. Arch. Neurol. 60, 1454–1456 (2003).
Pickering-Brown, S. M. et al. Inherited frontotemporal dementia in nine British families associated with intronic mutations in the tau gene. Brain 125, 732–751 (2002).
Chan, D. et al. The clinical profile of right temporal lobe atrophy. Brain 132, 1287–1298 (2009).
Josephs, K. A. et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 73, 1443–1450 (2009).
Josephs, K. A. et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol. Appl. Neurobiol. 30, 369–373 (2004).
Lipton, A. M., White, C. L. 3rd & Bigio, E. H. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta. Neuropathol. 108, 379–385 (2004).
Sampathu, D. M. et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am. J. Pathol. 169, 1343–1352 (2006).
Mackenzie, I. R. et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta. Neuropathol. (Berl.) 112, 539–549 (2006).
Cairns, N. J. et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta. Neuropathol. 114, 5–22 (2007).
Josephs, K. A., Whitwell, J. L., Jack, C. R., Parisi, J. E. & Dickson, D. W. Frontotemporal lobar degeneration without lobar atrophy. Arch. Neurol. 63, 1632–1638 (2006).
Whitwell, J. L., Jack, C. R. Jr, Senjem, M. L. & Josephs, K. A. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology 66, 102–104 (2006).
Mackenzie, I. R. et al. A harmonized classification system for FTLD-TDP pathology. Acta. Neuropathol. 122, 111–113 (2011).
Grossman, M. et al. TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch. Neurol. 64, 1449–1454 (2007).
Josephs, K. A., Stroh, A., Dugger, B. & Dickson, D. W. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta. Neuropathol. 118, 349–358 (2009).
Snowden, J., Neary, D. & Mann, D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta. Neuropathol. 114, 31–38 (2007).
Rohrer, J. D. et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology 75, 2204–2211 (2010).
Whitwell, J. L. et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology 75, 2212–2220 (2010).
Beck, J. et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 131, 706–720 (2008).
Whitwell, J. L. et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch. Neurol. 64, 371–376 (2007).
Le Ber, I. et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131, 732–746 (2008).
Hodges, J. R. et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain 133, 300–306 (2010).
Chan, D. et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann. Neurol. 49, 433–442 (2001).
Rosen, H. J. et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208 (2002).
Kim, E. J. et al. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry. 78, 1375–1378 (2007).
Grossman, M. et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch. Neurol. 64, 1601–1609 (2007).
Whitwell, J. L. et al. Voxel-based morphometry in tau-positive and tau-negative frontotemporal lobar degenerations. Neurodegener. Dis. 1, 225–230 (2004).
Pereira, J. M. et al. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology 72, 1653–1660 (2009).
Whitwell, J. L. et al. MRI correlates of protein deposition and disease severity in postmortem frontotemporal lobar degeneration. Neurodegener. Dis. 6, 106–117 (2009).
Cairns, N. J. et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63, 1376–1384 (2004).
Josephs, K. A. et al. Neurofilament inclusion body disease: a new proteinopathy? Brain 126, 2291–2303 (2003).
Kusaka, H., Matsumoto, S. & Imai, T. An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta. Neuropathol. 80, 660–665 (1990).
Munoz, D. G. et al. FUS pathology in basophilic inclusion body disease. Acta. Neuropathol. 118, 617–627 (2009).
Josephs, K. A. et al. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta. Neuropathol. 116, 159–167 (2008).
Mackenzie, I. R., Foti, D., Woulfe, J. & Hurwitz, T. A. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131, 1282–1293 (2008).
Josephs, K. A. et al. Caudate atrophy on MRI is a characteristic feature of FTLD-FUS. Eur. J. Neurol. 17, 969–975 (2010).
Seelaar, H. et al. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J. Neurol. 257, 747–753 (2010).
Urwin, H. et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta. Neuropathol. 120, 33–41 (2010).
Rohrer, J. D. et al. The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 82, 1405–1407 (2010).
Hu, W. T. et al. Alzheimer's disease and corticobasal degeneration presenting as corticobasal syndrome. Mov. Disord. 24, 1375–1379 (2009).
Knibb, J. A., Xuereb, J. H., Patterson, K. & Hodges, J. R. Clinical and pathological characterization of progressive aphasia. Ann. Neurol. 59, 156–165 (2006).
Shelley, B. P., Hodges, J. R., Kipps, C. M., Xuereb, J. H. & Bak, T. H. Is the pathology of corticobasal syndrome predictable in life? Mov. Disord. 24, 1593–1599 (2009).
Knopman, D. S. et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann. Neurol. 57, 480–488 (2005).
Piguet, O., Halliday, G. M., Creasey, H., Broe, G. A. & Kril, J. J. Frontotemporal dementia and dementia with Lewy bodies in a case-control study of Alzheimer's disease. Int. Psychogeriatr. 21, 688–695 (2009).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
Rabinovici, G. D. et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 68, 1205–1212 (2007).
Engler, H. et al. In vivo amyloid imaging with PET in frontotemporal dementia. Eur. J. Nucl. Med. Mol. Imaging 35, 100–106 (2008).
Lehmann, M. et al. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer's disease. J. Alzheimers. Dis. 20, 587–598 (2010).
Whitwell, J. L. et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol. Aging 32, 1531–1541 (2009).
Josephs, K. A. et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer's disease. Mov. Disord. 25, 1246–1252 (2010).
Hu, W. T. et al. Multimodal predictors for Alzheimer disease in nonfluent primary progressive aphasia. Neurology 75, 595–602 (2010).
Womack, K. B. et al. Temporoparietal hypometabolism in frontotemporal lobar degeneration and associated imaging diagnostic errors. Arch. Neurol. 68, 329–337 (2011).
Jellinger, K. A. et al. Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta. Neuropathol. 121, 267–277 (2010).
Lehmann, M. et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer's disease. Neurobiol. Aging 32, 1466–1476 (2009).
Whitwell, J. L. et al. Imaging correlates of posterior cortical atrophy. Neurobiol. Aging 28, 1051–1061 (2007).
Whitwell, J. L. et al. Distinct anatomical subtypes of the behavioral variant of frontotemporal dementia: a cluster analysis study. Brain 132, 2932–2946 (2009).
Bigio, E. H., Brown, D. F. & White, C. L. 3rd. Progressive supranuclear palsy with dementia: cortical pathology. J. Neuropathol. Exp. Neurol. 58, 359–364 (1999).
Ashburner, J. & Friston, K. J. Voxel-based morphometry--the methods. Neuroimage 11, 805–821 (2000).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Fodero-Tavoletti, M. T. et al. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer's disease. Brain 134, 1089–1100 (2011).
Whitwell, J. L. et al. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 77, 866–874 (2011).
Hu, W. T. et al. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology 75, 2079–2086 (2010).
Whitwell, J. L. et al. Neuroimaging signatures of frontotemporal dementia genetics: C90RF72, tau, progranulin and sporadics. Brain http://dx.doi.org/10.1093/brain/aws001
Whitwell, J. L. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 29, 9, 661–664 (2009).
Acknowledgements
K. A. Josephs and J. L. Whitwell are funded by NIH grants R01-DC010367, R01-AG037491 and R21-AG38736, and The Dana Foundation.
Author information
Authors and Affiliations
Contributions
J. L. Whitwell researched data for the article, provided substantial contribution to discussions of the content and wrote the article. K. A. Josephs provided substantial contribution to discussion of the content and reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Summary of regional imaging findings from group studies that assessed patterns of atrophy in pathologically or genetically confirmed FTLD-tau diseases (DOC 79 kb)
Supplementary Table 2
Summary of regional imaging findings from group studies that assessed patterns of atrophy in pathologically or genetically confirmed FTLD-TDP or FTLD-FUS diseases (DOC 76 kb)
Rights and permissions
About this article
Cite this article
Whitwell, J., Josephs, K. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat Rev Neurol 8, 131–142 (2012). https://doi.org/10.1038/nrneurol.2012.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.7
This article is cited by
-
Cellular and regional vulnerability in frontotemporal tauopathies
Acta Neuropathologica (2019)
-
Extended FTLD pedigree segregating a Belgian GRN-null mutation: neuropathological heterogeneity in one family
Alzheimer's Research & Therapy (2018)
-
Neuron loss and degeneration in the progression of TDP-43 in frontotemporal lobar degeneration
Acta Neuropathologica Communications (2017)
-
Imaging and fluid biomarkers in frontotemporal dementia
Nature Reviews Neurology (2017)
-
Emerging Diagnostic and Therapeutic Strategies for Tauopathies
Current Neurology and Neuroscience Reports (2017)