Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease with a rapid course, characterized by motor neuron dysfunction, leading to progressive disability and death. This Review, which is aimed at neurologists, psychologists and other health professionals who follow evidence-based practice relating to ALS and frontotemporal dementia (FTD), examines the neuropsychological evidence that has driven the reconceptualization of ALS as a spectrum disorder ranging from a pure motor phenotype to ALS–FTD. It focuses on changes in cognition and behaviour, which vary in severity across the spectrum: around 50% individuals with ALS are within the normal range, 15% meet the criteria for ALS–FTD, and the remaining 35% are in the mid-spectrum range with milder and more focal impairments. The cognitive impairments include deficits in verbal fluency, executive functions, social cognition and language, and apathy is the most prevalent behavioural change. The pattern and severity of cognitive and behavioural change predicts underlying regional cerebral dysfunction from brain imaging and post-mortem pathology. Our increased recognition of cognition and behaviour as part of the ALS phenotype has led to the development and standardization of assessment tools, which have been incorporated into research and clinical care. Measuring change over the course of the disease is vital for clinical trials, and neuropsychology is proving to be a biomarker for the earliest preclinical changes.
Key points
-
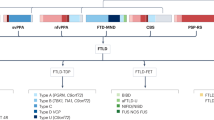
Cognitive and behavioural impairment in amyotrophic lateral sclerosis (ALS) is heterogeneous and represents a spectrum of changes from ALS to ALS–frontotemporal dementia, also referred to as ALS–frontotemporal spectrum disorder (ALS–FTSD).
-
Neuropsychology has been pivotal in identifying the mid-spectrum range of ALS–FTSD; executive, verbal fluency, social cognition and language impairments are common, and apathy is the most prevalent behavioural change.
-
Cerebral dysfunction underlying these impairments has been shown in both grey and white matter using a range of imaging techniques, and specific cognitive deficits were shown to predict TAR DNA-binding protein 43 pathology in specific brain regions.
-
Assessment tools including the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) and the ALS Cognitive Behavioural Screen (ALS-CBS) are well validated and standardized across different languages and are now incorporated into clinical trials.
-
This Review provides recommendations for neuropsychological assessment and intervention in ALS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marin, B. et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int. J. Epidemiol. 46, 57–74 (2017).
Cronin, S., Hardiman, O. & Traynor, B. J. Ethnic variation in the incidence of ALS: a systematic review. Neurology 68, 1002–1007 (2007).
Sennfält, S. et al. The path to diagnosis in ALS: delay, referrals, alternate diagnoses, and clinical progression. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 45–53 (2023).
Strong, M. J. et al. Amyotrophic lateral sclerosis – frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 153–174 (2017).
Beeldman, E. et al. The cognitive profile of ALS: a systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry 87, 611–619 (2016).
Lomen-Hoerth, C. et al. Are amyotrophic lateral sclerosis patients cognitively normal. Neurology 60, 1094–1097 (2003).
Montuschi, A. et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J. Neurol. Neurosurg. Psychiatry 86, 168–173 (2015).
Phukan, J. et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J. Neurol. Neurosurg. Psychiatry 83, 102–108 (2012).
Rakowicz, W. P. & Hodges, J. R. Dementia and aphasia in motor neuron disease: an underrecognised association? J. Neurol. Neurosurg. Psychiatry 65, 881–889 (1998).
Ringholz, G. M. et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65, 586–590 (2005).
Murphy, J. et al. Cognitive-behavioral screening reveals prevalent impairment in a large multicenter ALS cohort. Neurology 86, 813–820 (2016).
David, A. S. & Gillham, R. A. Neuropsychological study of motor neuron disease. Psychosomatics 27, 441–445 (1986).
Gallassi, R. et al. Cognitive impairment in motor neuron disease. Acta Neurol. Scand. 71, 480–484 (1985).
Gallassi, R. et al. Neuropsychological, electroencephalogram and brain computed tomography findings in motor neuron disease. Eur. Neurol. 29, 115–120 (1989).
Kew, J. J. M. et al. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis: a neuropsychological and positron emission tomography study. Brain 116, 1399–1423 (1993).
Ludolph, A. C. et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol. Scand. 85, 81–89 (1992).
Talbot, P. R. et al. Inter-relation between ‘classic’ motor neuron disease and frontotemporal dementia: neuropsychological and single photon emission computed tomography study. J. Neurol. Neurosurg. Psychiatry 58, 541–547 (1995).
Saxon, J. A. et al. Semantic dementia, progressive non-fluent aphasia and their association with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 711–712 (2017).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Ossenkoppele, R. et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138, 2732–2749 (2015).
Lima, M. et al. Neuropsychological assessment in the distinction between biomarker defined frontal-variant of Alzheimer’s disease and behavioral-variant of frontotemporal dementia. J. Alzheimers Dis. 91, 1303–1312 (2023).
Saxon, J. A. et al. Examining the language and behavioural profile in FTD and ALS-FTD. J. Neurol. Neurosurg. Psychiatry 88, 675–680 (2017).
Abrahams, S. et al. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38, 734–747 (2000).
Raaphorst, J. et al. The cognitive profile of amyotrophic lateral sclerosis: a meta-analysis. Amyotroph. Lateral Scler. 11, 27–37 (2010).
Abrahams, S. et al. Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 62, 464–472 (1997).
Murphy, J., Ahmed, F. & Lomen-Hoerth, C. The UCSF screening exam effectively screens cognitive and behavioral impairment in patients with ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 24–30 (2016).
Abrahams, S., Newton, J., Niven, E., Foley, J. & Bak, T. H. Screening for cognition and behaviour changes in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 9–14 (2014).
Beeldman, E. et al. The verbal fluency index: Dutch normative data for cognitive testing in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 388–391 (2014).
Abrahams, S. et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119, 2105–2120 (1996).
Abrahams, S. et al. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain 127, 1507–1517 (2004).
Abrahams, S. et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J. Neurol. 252, 321–331 (2005).
Baddeley, A. D. Working Memory (Oxford Univ. Press, 1986).
Pettit, L. D. et al. Executive deficits not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain 136, 3290–3304 (2013).
Libon, D. et al. Deficits in concept formation in amyotrophic lateral sclerosis. Neuropsychology 26, 422–429 (2012).
Lillo, P., Savage, S., Mioshi, E., Kiernan, M. C. & Hodges, J. R. Amyotrophic lateral sclerosis and frontotemporal dementia: a behavioural and cognitive continuum. Amyotroph. Lateral Scler. 13, 102–109 (2012).
Girardi, A., Macpherson, S. E. & Abrahams, S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology 25, 53–65 (2011).
van der Hulst, E. J., Bak, T. H. & Abrahams, S. Impaired affective and cognitive theory of mind and behavioural change in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 86, 1208–1215 (2015).
Cavallo, M. et al. Evidence of social understanding impairment in patients with amyotrophic lateral sclerosis. PLoS ONE 6, e25948 (2011).
Bora, E. Meta-analysis of social cognition in amyotrophic lateral sclerosis. Cortex 88, 1–7 (2017).
Lillo, P. et al. Inside minds, beneath diseases: social cognition in amyotrophic lateral sclerosis-frontotemporal spectrum disorder. J. Neurol. Neurosurg. Psychiatry 91, 1279–1282 (2020).
Palumbo, F. et al. Social cognition deficits in amyotrophic lateral sclerosis: a pilot cross-sectional population-based study. Eur. J. Neurol. 29, 2211–2219 (2022).
Burke, T. et al. Measurement of social cognition in amyotrophic lateral sclerosis: a population based study. PLoS ONE 11, e0160850 (2016).
Aiello, E. N. et al. Validity and diagnostics of the Reading the Mind in the Eyes Test (RMET) in non-demented amyotrophic lateral sclerosis (ALS) patients. Front. Psychol. 13, 1031841 (2022).
Burke, T. et al. The reading the mind in the eyes test short form (A & B): validation and outcomes in an amyotrophic lateral sclerosis cohort. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 380–388 (2020).
Trojsi, F. et al. Resting state fMRI correlates of theory of mind impairment in amyotrophic lateral sclerosis. Cortex 97, 1–16 (2017).
Lulé, D. et al. Clinicoanatomical substrates of selfish behaviour in amyotrophic lateral sclerosis – an observational cohort study. Cortex 146, 261–270 (2022).
Taylor, L. J. et al. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry 84, 494–498 (2013).
Pinto-Grau, M. et al. Patterns of language impairment in early amyotrophic lateral sclerosis. Neurol. Clin. Pract. 11, e634–e644 (2021).
Burke, T. et al. Visual encoding, consolidation, and retrieval in amyotrophic lateral sclerosis: executive function as a mediator, and predictor of performance. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 193–201 (2017).
Iazzolino, B. et al. Differential neuropsychological profile of patients with amyotrophic lateral sclerosis with and without C9orf72 mutation. Neurology 96, e141–e152 (2021).
Raaphorst, J. et al. Prose memory impairment in amyotrophic lateral sclerosis patients is related to hippocampus volume. Eur. J. Neurol. 22, 547–554 (2015).
Christidi, F. et al. Uncinate fasciculus microstructure and verbal episodic memory in amyotrophic lateral sclerosis: a diffusion tensor imaging and neuropsychological study. Brain Imaging Behav. 8, 497–505 (2014).
Lulé, D. et al. Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex 101, 163–171 (2018).
Crockford, C. et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 91, e1370–e1380 (2018).
Gibbons, Z. C. et al. Behaviour in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 9, 67–74 (2008).
Lillo, P. et al. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 12, 45–51 (2011).
Radakovic, R. & Abrahams, S. Multidimensional apathy: evidence from neurodegenerative disease. Curr. Opin. Behav. Sci. 22, 42–49 (2018).
Radakovic, R. et al. Multidimensional apathy in ALS: validation of the dimensional apathy scale. J. Neurol. Neurosurg. Psychiatry 87, 663–669 (2016).
Caga, J. et al. Apathy is associated with parietal cortical-subcortical dysfunction in ALS. Cortex 145, 341–349 (2021).
Tsujimoto, M. et al. Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J. Neurol. Sci. 307, 34–40 (2011).
Femiano, C. et al. Apathy is correlated with widespread diffusion tensor imaging (DTI) impairment in amyotrophic lateral sclerosis. Behav. Neurol. 2018, 2635202 (2018).
Gregory, J. M. et al. Executive, language and fluency dysfunction are markers of localised TDP-43 cerebral pathology in non-demented ALS. J. Neurol. Neurosurg. Psychiatry 91, 149–157 (2020).
Radakovic, R. et al. Multidimensional apathy and executive dysfunction in amyotrophic lateral sclerosis. Cortex 94, 142–151 (2017).
Olney, R. K. et al. The effects of executive and behavioral dysfunction on the course of ALS. Neurology 65, 1774–1777 (2005).
Burke, T., Elamin, M., Galvin, M., Hardiman, O. & Pender, N. Caregiver burden in amyotrophic lateral sclerosis: a cross-sectional investigation of predictors. J. Neurol. 262, 1526–1532 (2015).
Chio, A. et al. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. Eur. J. Neurol. 17, 1298–1303 (2010).
Hsieh, S., Schubert, S., Hoon, C., Mioshi, E. & Hodges, J. R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 36, 242–250 (2013).
Woolley, S. C. et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioral Screen (ALS-CBS). Amyotroph. Lateral Scler. 11, 303–311 (2010).
De Icaza Valenzuela, M. M. et al. Validation of The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) in behavioural variant frontotemporal dementia and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 36, 1576–1587 (2021).
Kourtesis, P. et al. The Edinburgh cognitive and behavioral amyotrophic lateral sclerosis screen (ECAS): sensitivity in differentiating between ALS and Alzheimer’s disease in a Greek population. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 78–85 (2020).
Kourtesis, P., Margioti, E., Demenega, C., Christidi, F. & Abrahams, S. A comparison of the Greek ACE-III, M-ACE, ACE-R, MMSE, and ECAS in the Assessment and Identification of Alzheimer’s disease. J. Int. Neuropsychol. Soc. 26, 825–834 (2020).
Lulé, D. et al. Screening for cognitive function in complete immobility using brain–machine interfaces: a proof of principle study. Front. Neurosci. 12, 517 (2018).
Niven, E. et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): a cognitive tool for motor disorders. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 172–179 (2015).
Aiello, E. N. et al. The diagnostic value of the Italian version of the Edinburgh Cognitive and Behavioral ALS Screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 527–531 (2022).
Pinto-Grau, M. et al. Screening for cognitive dysfunction in ALS: validation of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) using age and education adjusted normative data. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 99–106 (2017).
Poletti, B. et al. The validation of the Italian Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 489–498 (2016).
Lulé, D. et al. The Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 16–23 (2015).
Saxon, J. A. et al. The Edinburgh Cognitive and Behavioral ALS Screen (ECAS) in frontotemporal dementia. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 606–613 (2020).
Christodoulou, G. et al. Telephone based cognitive-behavioral screening for frontotemporal changes in patients with amyotrophic lateral sclerosis (ALS). Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 482–488 (2016).
Kacem, I. et al. Arabic adaptation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis screen (ECAS-AR). Rev. Neurol. 178, 817–825 (2022).
Watanabe, Y., Ogino, M., Ichikawa, H., Hanajima, R. & Nakashima, K. The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) for Japanese ALS and FTD patients. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 66–72 (2021).
Albertyn, C. H. et al. Adaptation and norming of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis screen (ECAS) for three language groups in South Africa. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 532–541 (2022).
Mora, J. S. et al. Spanish adaptation of the Edinburgh Cognitive and Behavioral Amyotrophic Lateral Sclerosis screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 74–79 (2018).
Elamin, M. et al. Identifying behavioural changes in ALS: validation of the Beaumont Behavioural Inventory (BBI). Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 68–73 (2017).
Neary, D. et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554 (1998).
Radakovic, R., Davenport, R., Starr, J. M. & Abrahams, S. Apathy dimensions in Parkinson’s disease. Int. J. Geriatr. Psychiatry 33, 151–158 (2018).
Radakovic, R., Starr, J. M. & Abrahams, S. A novel assessment and profiling of multidimensional apathy in Alzheimer’s disease. J. Alzheimers Dis. 60, 57–67 (2017).
Trojsi, F. et al. Microstructural correlates of Edinburgh Cognitive and Behavioural ALS Screen (ECAS) changes in amyotrophic lateral sclerosis. Psychiatry Res. Neuroimaging 288, 67–75 (2019).
Chenji, S. et al. Neuroanatomical associations of the Edinburgh Cognitive and Behavioural ALS screen (ECAS). Brain imaging Behav. 15, 1641–1654 (2021).
Keller, J. et al. Functional reorganization during cognitive function tasks in patients with amyotrophic lateral sclerosis. Brain Imaging Behav. 12, 771–784 (2018).
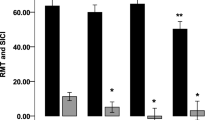
Higashihara, M. et al. Association of cortical hyperexcitability and cognitive impairment in patients with amyotrophic lateral sclerosis. Neurology 96, e2090–e2097 (2021).
Verde, F., Otto, M. & Silani, V. Neurofilament light chain as biomarker for amyotrophic lateral sclerosis and frontotemporal dementia. Front. Neurosci. 15, 679199 (2021).
Scherling, C. S. et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann. Neurol. 75, 116–126 (2014).
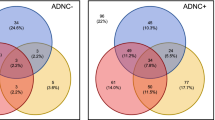
Illán-Gala, I. et al. CSF sAPPβ, YKL-40, and NfL along the ALS-FTD spectrum. Neurology 91, e1619–e1628 (2018).
van der Ende, E. L. et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol. 18, 1103–1111 (2019).
Chiò, A. et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 93, e984–e994 (2019).
Roche, J. C. et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 135, 847–852 (2012).
Beeldman, E. et al. Progression of cognitive and behavioural impairment in early amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 91, 779–780 (2020).
Bersano, E. et al. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: a longitudinal study. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 373–379 (2020).
Elamin, M. et al. Cognitive changes predict functional decline in ALS: a population-based longuitudinal study. Neurology 80, 1590–1596 (2013).
Elamin, M. et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 76, 1263–1269 (2011).
Ye, S., Jin, P., Chen, L., Zhang, N. & Fan, D. Prognosis of amyotrophic lateral sclerosis with cognitive and behavioural changes based on a sixty-month longitudinal follow-up. PLoS ONE 16, e0253279 (2021).
Aiello, E. N. et al. Reliable change indices for the Italian Edinburgh Cognitive and Behavioral ALS Screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 339–342 (2022).
Crockford, C. et al. Development of parallel versions of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 115–123 (2015).
Crockford, C. et al. Measuring reliable change in cognition using the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 65–73 (2018).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Byrne, S. et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 11, 232–240 (2012).
Chiò, A. et al. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain 135, 784–793 (2012).
Lulé, D. E. et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers – a developmental disorder. J. Neurol. Neurosurg. Psychiatry 91, 1195–1200 (2020).
Benatar, M., Turner, M. R. & Wuu, J. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 303–309 (2019).
Barker, M. S. et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain 145, 1079–1097 (2022).
Lingor, P. et al. ROCK-ALS: protocol for a randomized, placebo-controlled, double-blind phase IIa trial of safety, tolerability and efficacy of the Rho kinase (ROCK) inhibitor fasudil in amyotrophic lateral sclerosis. Front. Neurol. 10, 293 (2019).
Henderson, R. D. et al. Phase 1b dose-escalation, safety, and pharmacokinetic study of IC14, a monoclonal antibody against CD14, for the treatment of amyotrophic lateral sclerosis. Medicine 100, e27421 (2021).
Beswick, E. et al. A systematic review of neuropsychiatric and cognitive assessments used in clinical trials for amyotrophic lateral sclerosis. J. Neurol. 268, 4510–4521 (2021).
National Institute for Health and Care Excellence. Motor neurone disease: assessment and management. NICE https://www.nice.org.uk/guidance/ng42 (2019).
Gray, D. & Abrahams, S. International evaluation of current practices in cognitive assessment for motor neurone disease. Br. J. Neurosci. Nurs. 18, 38–44 (2022).
Hodgins, F., Bell, S. & Abrahams, S. Factors influencing the implementation of cognitive and behavioural screening in motor neurone disease. Br. J. Neurosci. Nurs. 13, 115–119 (2018).
Hodgins, F., Mulhern, S. & Abrahams, S. The clinical impact of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) and neuropsychological intervention in routine ALS care. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 92–99 (2020).
Crockford, C., Stockton, C. & Abrahams, S. Clinicians’ attitudes towards cognitive and behavioural screening in motor neurone disease. Br. J. Neurosci. Nurs. 13, 116–123 (2017).
Gould, R. L. et al. A randomised controlled trial of acceptance and commitment therapy plus usual care compared to usual care alone for improving psychological health in people with motor neuron disease (COMMEND): study protocol. BMC Neurol. 22, 431 (2022).
McMackin, R. et al. Cognitive network hyperactivation and motor cortex decline correlate with ALS prognosis. Neurobiol. Aging 104, 57–70 (2021).
Ratti, E. et al. Regional prefrontal cortical atrophy predicts specific cognitive-behavioral symptoms in ALS-FTD. Brain Imaging Behav. 15, 2540–2551 (2021).
Witiuk, K. et al. Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J. Neurosci. 34, 14260–14271 (2014).
Canosa, A. et al. Brain metabolic correlates of apathy in amyotrophic lateral sclerosis: an 18F-FDG-positron emission tomography stud. Eur. J. Neurol. 28, 745–753 (2021).
Carluer, L. et al. Neural substrate of cognitive theory of mind impairment in amyotrophic lateral sclerosis. Cortex 65, 19–30 (2015).
Castelnovo, V. et al. Progression of brain functional connectivity and frontal cognitive dysfunction in ALS. Neuroimage Clin. 28, 102509 (2020).
Wicks, P. et al. Neuronal loss associated with cognitive performance in amyotrophic lateral sclerosis: an (11C)-flumazenil PET study. Amyotroph. Lateral Scler. 9, 43–49 (2008).
Yabe, I. et al. Writing errors in ALS related to loss of neuronal integrity in the anterior cingulate gyrus. J. Neurol. Sci. 315, 55–59 (2012).
Tan, H. H. G. et al. MRI clustering reveals three ALS subtypes with unique neurodegeneration patterns. Ann. Neurol. 92, 1030–1045 (2022).
Canosa, A. et al. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology 86, 44–49 (2016).
Ye, S. et al. Cortical thickness and cognitive impairment in patients with amyotrophic lateral sclerosis. Beijing Da Xue Xue Bao Yi Xue Ban. 54, 1158–1162 (2022).
Consonni, M., Cappa, S. F., Dalla Bella, E., Contarino, V. E. & Lauria, G. Cortical correlates of behavioural change in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 380–386 (2019).
Trojsi, F. et al. Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: a 3T high angular resolution diffusion imaging (HARDI) study. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 553–561 (2013).
Agosta, F. et al. Structural brain correlates of cognitive and behavioral impairment in MND. Hum. Brain Mapp. 37, 1614–1626 (2016).
Crespi, C. et al. Microstructural white matter correlates of emotion recognition impairment in amyotrophic lateral sclerosis. Cortex 53, 1–8 (2014).
Woolley, S. C., Zhang, Y., Schuff, N., Weiner, M. W. & Katz, J. S. Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph. Lateral Scler. 12, 52–58 (2011).
Goldstein, L. H. & Abrahams, S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 12, 368–380 (2013).
Pender, N., Pinto-Grau, M. & Hardiman, O. Cognitive and behavioural impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 33, 649–654 (2020).
Acknowledgements
The work based at the University of Edinburgh focusing on the Edinburgh Cognitive and Behavioural ALS Screen and the Dimensional Apathy Scale was funded by the Motor Neurone Disease Association, MND Scotland and the ALS Association. The work described at Edinburgh was undertaken with the help of the MND-Cognition research team T. Bak, J. Newton, R. Radakovic, C. Crockford, C. McHutchison, D. Gray, L. Pettitt, D. Van Der Hulst, A. Girardi, M. Cavallo and E. Niven. The work is also supported by the Euan Macdonald Centre for MND Research and the Anne Rowling Regenerative Neurology Clinic. In addition to the above the author thanks her collaborators including O. Hardiman, A. Al Chalabi, Z. Simmons, M. Benatar, S. MacPherson, S. Pal, S. Chandran, L. Goldstein and N. Leigh. Most importantly, the author thanks all the people with neurodegenerative disease and their families who have helped with this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.A. is one of the authors of the Edinburgh Cognitive and Behavioural ALS Screen and the Dimensional Apathy Scale.
Peer review
Peer review information
Nature Reviews Neurology thanks V. Silani, M. Strong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abrahams, S. Neuropsychological impairment in amyotrophic lateral sclerosis–frontotemporal spectrum disorder. Nat Rev Neurol 19, 655–667 (2023). https://doi.org/10.1038/s41582-023-00878-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00878-z
This article is cited by
-
Quality of life, cognitive and behavioural impairment in people with motor neuron disease: a systematic review
Quality of Life Research (2024)